Vancomycin-resistant Staphylococcus aureus: Difference between revisions
Hardik Patel (talk | contribs) No edit summary |
Hardik Patel (talk | contribs) |
||
| Line 16: | Line 16: | ||
http://www.cdc.gov/ncidod/dhqp/ar_visavrsa_labFAQ.html | http://www.cdc.gov/ncidod/dhqp/ar_visavrsa_labFAQ.html | ||
===== References ===== | ===== References ===== | ||
Revision as of 15:02, 17 December 2012
|
Vancomycin-resistant Staphylococcus aureus Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Vancomycin-resistant Staphylococcus aureus On the Web |
|
American Roentgen Ray Society Images of Vancomycin-resistant Staphylococcus aureus |
|
Directions to Hospitals Treating Vancomycin-resistant Staphylococcus aureus |
|
Risk calculators and risk factors for Vancomycin-resistant Staphylococcus aureus |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
References
http://www.cdc.gov/ncidod/dhqp/ar_visavrsa.html
References
http://www.cdc.gov/ncidod/dhqp/ar_visavrsa_FAQ.html#3
References
http://www.cdc.gov/ncidod/dhqp/ar_visavrsa_FAQ.html#3
References
http://www.cdc.gov/ncidod/dhqp/ar_visavrsa_labFAQ.html
References
http://www.cdc.gov/ncidod/dhqp/ar_visavrsa_FAQ.html#3 http://www.cdc.gov/ncidod/dhqp/ar_visavrsa_FAQ.html#1
Natural History
References
References
Laboratory Findings
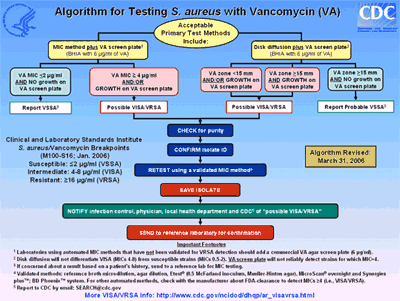
All laboratories should develop a step-by-step problem-solving procedure or algorithm for detecting VISA/VRSA that is specific for their laboratory. A sample algorithm is available here and highlights the recommended testing methodologies for detecting VISA/VRSA and actions based on results.
Not all susceptibility testing methods detect VISA and VRSA isolates. Three out of six confirmed VRSA isolates were not reliably detected by automated testing systems in a recent report. Subsequently, some manufacturers have optimized their systems for VRSA detection, so laboratories should check with manufacturers to determine if their system has FDA clearance for VRSA detection. VRSA are detected by reference broth microdilution, agar dilution, Etest®, MicroScan® overnight and Synergies plus™; BD Phoenix™ system, disk diffusion, and the vancomycin screen agar plate [brain heart infusion (BHI) agar containing 6 µg/ml of vancomycin].
VISA isolates are not detected by disk diffusion. Methods that typically detect VISA are non-automated MIC methods including reference broth microdilution, agar dilution, and Etest® using a 0.5 McFarland standard to prepare inoculum. Automated methods and vancomycin screen agar plates usually detect VISA for which the vancomycin MICs are 8 µg/ml, but further studies are need to define the level of sensitivity of these methods for S. aureus for which the vancomycin MICs are 4 µg/ml.
Electrolyte and Biomarker Studies
References
http://www.cdc.gov/ncidod/dhqp/ar_visavrsa_algo.html http://www.cdc.gov/ncidod/dhqp/ar_visavrsa_labFAQ.html
Treatment
VISA and VRSA cannot be successfully treated with vancomycin because these organisms are no longer susceptibile to vancomycin. However, to date, all VISA and VRSA isolates have been susceptible to other Food and Drug Administration (FDA) approved drugs.
Pharmacotherapy
References
http://www.cdc.gov/ncidod/dhqp/ar_visavrsa.html
Primary Prevention
Use of appropriate infection control practices (such as wearing gloves before and after contact with infectious body substances and adherence to hand hygiene) by healthcare personnel can reduce the spread of VISA and VRSA.
References
http://www.cdc.gov/ncidod/dhqp/ar_visavrsa_FAQ.html#3
Secondary Prevention
Because VISA and VRSA are only part of the larger problem of antimicrobial resistance in healthcare settings, CDC has started a Campaign to Prevent Antimicrobial Resistance. The campaign centers around four strategies that clinicians can use to prevent antimicrobial resistance: prevent infections; diagnose and treat infections effectively; use antimicrobials wisely; and prevent transmission. A series of evidence-based steps are described that can reduce the development and spread of resistant organisms such as VISA and VRSA.
References
http://www.cdc.gov/ncidod/dhqp/ar_visavrsa_FAQ.html#3
Additional Resources
- ^ Chang S, Sievert DM, Hageman JC, Boulton ML, Tenover FC, Downes FP, Shah S, Rudrik JT, Pupp GR, Brown WJ, Cardo D, Fridkin SK. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med 2003;348:1342-7. PMID 12672861.