Trifluridine and tipiracil
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Allison Tu [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Trifluridine and tipiracil is a combination of a nucleoside metabolic inhibitor and a thymidine phosphorylase inhibitor that is FDA approved for the treatment of patients with metastatic colorectal cancer who have been previously treated with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, an anti-VEGF biological therapy, and if RAS wild-type, an anti-EGFR therapy. Common adverse reactions include fatigue, nausea, diarrhea, decreased appetite, abdominal pain, anemia, neutropenia, thrombocytopenia, weakness, and fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Trifluridine and tipiracil is indicated for the treatment of patients with metastatic colorectal cancer who have been previously treated with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, an anti-VEGF biological therapy, and if RAS wild-type, an anti-EGFR therapy.
Dosing Information
- The recommended starting dose of trifluridine and tipiracil is 35 mg/m2 up to a maximum of 80 mg per dose (based on the trifluridine component) orally twice daily within one hour of completion of morning and evening meals on Days 1 through 5 and Days 8 through 12 of each 28-day cycle until disease progression or unacceptable toxicity.
- Round dose to the nearest 5 mg increment.
- Do not take additional doses to make up for missed or held doses.
- Do not initiate the cycle of trifluridine and tipiracil until:
- Absolute neutrophil count (ANC) is greater than or equal to 1,500/mm3 or febrile neutropenia is resolved
- Platelets are greater than or equal to 75,000/mm3
- Grade 3 or 4 non-hematological adverse reactions are resolved to Grade 0 or 1
- Within a treatment cycle, withhold trifluridine and tipiracil for any of the following:
- Absolute neutrophil count (ANC) less than 500/mm3 or febrile neutropenia
- Platelets less than 50,000/mm3
- Grade 3 or 4 non-hematological adverse reactions
- After recovery, resume trifluridine and tipiracil after reducing the dose by 5 mg/m2/dose from the previous dose level, if the following occur:
- Febrile neutropenia
- Uncomplicated Grade 4 neutropenia (which has recovered to greater than or equal to 1,500/mm3) or thrombocytopenia (which has recovered to greater than or equal to 75,000/mm3) that results in more than 1 week delay in start of next cycle.
- Non-hematologic Grade 3 or Grade 4 adverse reaction except for Grade 3 nausea and/or vomiting controlled by antiemetic therapy or Grade 3 diarrhea responsive to antidiarrheal medication.
- A maximum of 3 dose reductions are permitted to a minimum dose of 20 mg/m2 twice daily. Do not escalate trifluridine and tipiracil dose after it has been reduced.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Trifluridine and tipiracil in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Trifluridine and tipiracil in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Trifluridine and tipiracil FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Trifluridine and tipiracil in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Trifluridine and tipiracil in pediatric patients.
Contraindications
There is limited information regarding Trifluridine and tipiracil Contraindications in the drug label.
Warnings
- Severe Myelosuppression
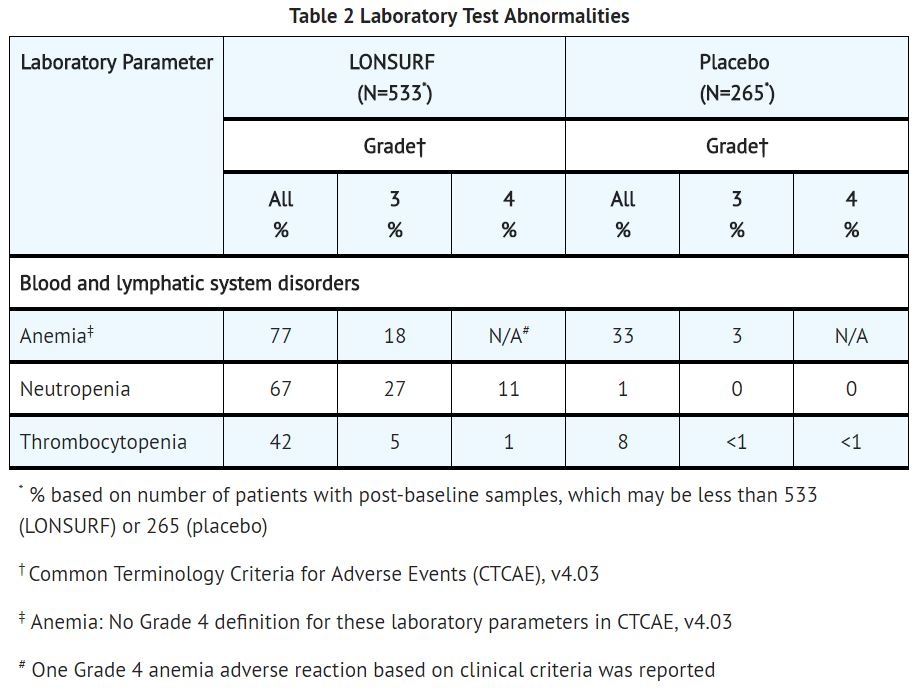
- In Study 1, trifluridine and tipiracil caused severe and life-threatening myelosuppression (Grade 3-4) consisting of anemia (18%), neutropenia (38%), thrombocytopenia (5%) and febrile neutropenia (3.8%). One patient (0.2%) died due to neutropenic infection. In Study 1, 9.4% of trifluridine and tipiracil-treated patients received granulocyte-colony stimulating factors.
- Obtain complete blood counts prior to and on Day 15 of each cycle of trifluridine and tipiracil and more frequently as clinically indicated. Withhold trifluridine and tipiracil for febrile neutropenia, Grade 4 neutropenia, or platelets less than 50,000/mm3. Upon recovery resume trifluridine and tipiracil at a reduced dose.
- Embryo-Fetal Toxicity
- Based on animal studies and its mechanism of action, trifluridine and tipiracil can cause fetal harm when administered to a pregnant woman. Trifluridine/tipiracil caused embryo-fetal lethality and embryo-fetal toxicity in pregnant rats when orally administered during gestation at dose levels resulting in exposures lower than those achieved at the recommended dose of 35 mg/m2 twice daily.
- Advise pregnant women of the potential risk to the fetus. Advise females of reproductive potential to use effective contraception during treatment with trifluridine and tipiracil.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below are from Study 1, a randomized (2:1), double-blind, placebo-controlled trial in which 533 patients (median age 63 years; 61% men; 57% White, 35% Asian, 1% Black) with previously treated metastatic colorectal cancer received trifluridine and tipiracil as a single agent at a dose of 35 mg/m2/dose administered twice daily on Days 1 through 5 and Days 8 through 12 of each 28-day cycle. The mean duration of trifluridine and tipiracil therapy was 12.7 weeks.
The most common adverse drug reactions or laboratory abnormalities (all grades and greater than or equal to 10% in incidence) in patients treated with trifluridine and tipiracil at a rate that exceeds the rate in patients receiving placebo were anemia, neutropenia, asthenia/fatigue, nausea, thrombocytopenia, decreased appetite, diarrhea, vomiting, abdominal pain, and pyrexia.
In Study 1, 3.6% of patients discontinued trifluridine and tipiracil for an adverse event and 13.7% of patients required a dose reduction. The most common adverse reactions leading to dose reduction were neutropenia, anemia, febrile neutropenia, fatigue, and diarrhea.
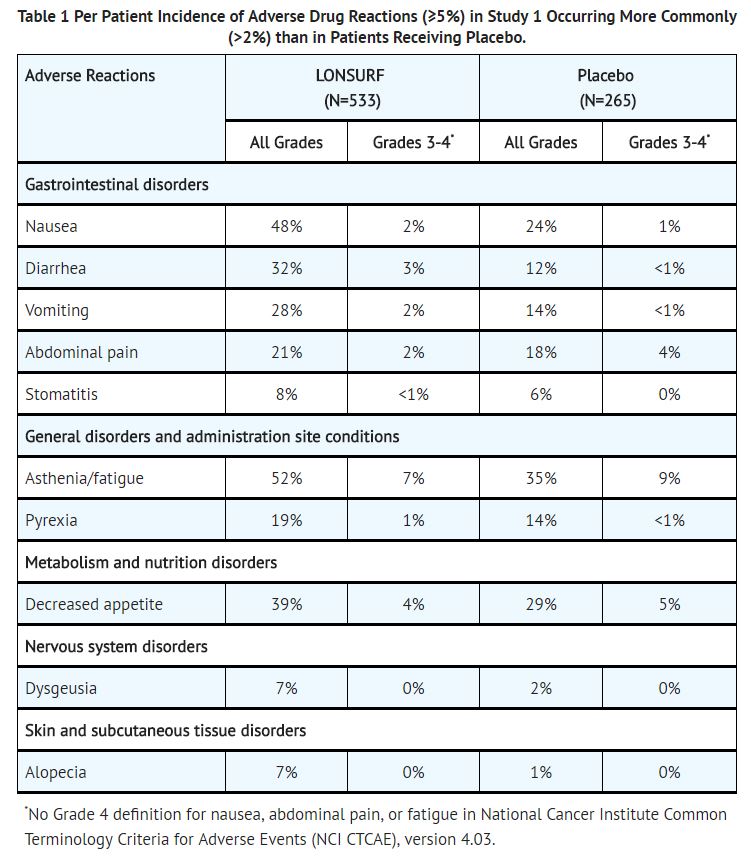
In Study 1, infections occurred more frequently in trifluridine and tipiracil-treated patients (27%) compared to those receiving placebo (15%). The most commonly reported infections which occurred more frequently in trifluridine and tipiracil-treated patients were nasopharyngitis (4% versus 2%), and urinary tract infections (4% versus 2%).
In Study 1, pulmonary emboli occurred more frequently in trifluridine and tipiracil-treated patients (2%) compared to no patients on placebo.
Additional Clinical Experience:
Interstitial lung disease was reported in fifteen (0.2%) patients, three of which were fatal, among approximately 7,000 patients exposed to trifluridine and tipiracil in clinical studies and clinical practice settings in Asia.
Postmarketing Experience
There is limited information regarding Trifluridine and tipiracil Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Trifluridine and tipiracil Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Risk Summary
- Based on animal data and its mechanism of action, trifluridine and tipiracil can cause fetal harm. trifluridine and tipiracil caused embryo-fetal lethality and embryo-fetal toxicity in pregnant rats when given during gestation at doses resulting in exposures lower than or similar to exposures at the recommended dose in humans.
- There are no available data on trifluridine and tipiracil exposure in pregnant women. Advise pregnant women of the potential risk to a fetus.
- In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
- Data
- Animal data
- Trifluridine/tipiracil was administered orally once daily to female rats during organogenesis at dose levels of 15, 50, and 150 mg/kg [trifluridine (FTD) equivalent].
- Decreased fetal weight was observed at FTD doses greater than or equal to 50 mg/kg (approximately 0.33 times the exposure at the clinical dose of 35 mg/m2 twice daily).
- At the FTD dose of 150 mg/kg (approximately 0.92 times the FTD exposure at the clinical dose of 35 mg/m2 twice daily) embryolethality and structural anomalies (kinked tail, cleft palate, ectrodactyly, anasarca, alterations in great vessels, and skeletal anomalies) were observed.
- Animal data
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Trifluridine and tipiracil in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Trifluridine and tipiracil during labor and delivery.
Nursing Mothers
- Risk Summary
- It is not known whether trifluridine and tipiracil or its metabolites are present in human milk.
- In nursing rats, trifluridine and tipiracil or their metabolites were present in breast milk. There are no data to assess the effects of trifluridine and tipiracil or its metabolites on the breastfed infant or the effects on milk production.
- Because of the potential for serious adverse reactions in breastfeeding infants, advise women not to breastfeed during treatment with trifluridine and tipiracil and for one day following the final dose.
- Data
- Radioactivity was excreted in the milk of nursing rats dosed with trifluridine/tipiracil containing 14C-FTD or 14C-tipiracil (TPI).
- Levels of FTD-derived radioactivity were as high as approximately 50% of the exposure in maternal plasma an hour after dosing with trifluridine/tipiracil and were approximately the same as those in maternal plasma for up to 12 hours following dosing.
- Exposure to TPI-derived radioactivity was higher in milk than in maternal plasma beginning 2 hours after dosing and continuing for at least 12 hours following administration of trifuridine/tipiracil.
Pediatric Use
Safety and effectiveness of trifluridine and tipiracil in pediatric patients have not been established.
Animal Data: Dental toxicity including whitening, breakage, and malocclusion (degeneration and disarrangement in the ameloblasts, papillary layer cells and odontoblasts) were observed in rats treated with trifluridine/tipiracil at doses greater than or equal to 50 mg/kg (approximately 0.33 times the exposure at the clinical dose of 35 mg/m2 twice daily).
Geriatic Use
In Study 1, 533 patients received trifluridine and tipiracil; 44% were 65 years of age or over, while 7% were 75 and over. No overall differences in effectiveness were observed in patients 65 or older versus younger patients, and no adjustment is recommended for the starting dose of trifluridine and tipiracil based on age.
Patients 65 years of age or older who received trifluridine and tipiracil had a higher incidence of the following compared to patients younger than 65 years: Grade 3 or 4 neutropenia (48% vs 30%), Grade 3 anemia (26% vs 12%, and Grade 3 or 4 thrombocytopenia (9% vs 2%).
Gender
There is no FDA guidance on the use of Trifluridine and tipiracil with respect to specific gender populations.
Race
There were no clinically meaningful differences in Study 1 between Western and Asian subgroups with respect to overall incidence of adverse events or ≥ Grade 3 adverse events in either the trifluridine and tipiracil or placebo groups.
Renal Impairment
No dedicated clinical studies have been conducted to evaluate the effect of renal impairment on the pharmacokinetics of trifluridine and tipiracil.
In Study 1, patients with moderate renal impairment (CrCl = 30 to 59 mL/min, n= 47) had a higher incidence (difference of at least 5%) of ≥ Grade 3 adverse events, serious adverse events, and dose delays and reductions compared to patients with normal renal function (CrCl ≥ 90 mL/min, n= 306) or patients with mild renal impairment (CrCl = 60 to 89 mL/min, n= 178).
No adjustment to the starting dose of trifluridine and tipiracil is recommended in patients with mild or moderate renal impairment (CrCl of 30 to 89 mL/min); however patients with moderate renal impairment may require dose modification for increased toxicity. Patients with severe renal impairment (CrCl < 30 mL/min) were not studied.
Hepatic Impairment
In a pharmacokinetic trial comparing 10 patients with mild hepatic impairment (total bilirubin less than or equal to the upper limit of normal (ULN) and AST greater than ULN or TB less than 1 to 1.5 times ULN and any AST) and 6 patients with moderate hepatic impairment (total bilirubin greater than 1.5 to 3 times ULN and any AST) to 8 patients with normal hepatic function, no clinically important differences in the mean exposures of trifluridine and tipiracil were observed. Five of 6 patients with moderate hepatic impairment experienced Grade 3 or 4 increased bilirubin levels. Patients with severe hepatic impairment (total bilirubin greater than 3 times ULN and any AST) were not studied. No adjustment to the starting dose of trifluridine and tipiracil is recommended for patients with mild hepatic impairment. Do not initiate trifluridine and tipiracil in patients with baseline moderate or severe (total bilirubin greater than 1.5 times ULN and any AST) hepatic impairment.
Females of Reproductive Potential and Males
Contraception:
- Females
- Trifluridine and tipiracil can cause fetal harm when administered to a pregnant woman.
- Advise females of reproductive potential to use effective contraception during treatment.
- Males
- Because of the potential for genotoxicity, advise males with female partners of reproductive potential to use condoms during treatment with trifluridine and tipiracil and for at least 3 months after the final dose.
Immunocompromised Patients
There is no FDA guidance one the use of Trifluridine and tipiracil in patients who are immunocompromised.
Administration and Monitoring
Administration
- Trifluridine and tipiracil is a cytotoxic drug. Follow applicable special handling and disposal procedures.
- Do not initiate the cycle of trifluridine and tipiracil until:
- Absolute neutrophil count (ANC) is greater than or equal to 1,500/mm3 or febrile neutropenia is resolved
- Platelets are greater than or equal to 75,000/mm3
- Grade 3 or 4 non-hematological adverse reactions are resolved to Grade 0 or 1
- Within a treatment cycle, withhold trifluridine and tipiracil for any of the following:
- Absolute neutrophil count (ANC) less than 500/mm3 or febrile neutropenia
- Platelets less than 50,000/mm3
- Grade 3 or 4 non-hematological adverse reactions
- After recovery, resume trifluridine and tipiracil after reducing the dose by 5 mg/m2/dose from the previous dose level, if the following occur:
- Febrile neutropenia
- Uncomplicated Grade 4 neutropenia (which has recovered to greater than or equal to 1,500/mm3) or thrombocytopenia (which has recovered to greater than or equal to 75,000/mm3) that results in more than 1 week delay in start of next cycle
- Non-hematologic Grade 3 or Grade 4 adverse reaction except for Grade 3 nausea and/or vomiting controlled by antiemetic therapy or Grade 3 diarrhea responsive to antidiarrheal medication
- A maximum of 3 dose reductions are permitted to a minimum dose of 20 mg/m2 twice daily. Do not escalate trifluridine and tipiracil dose after it has been reduced.
Monitoring
There is limited information regarding Trifluridine and tipiracil Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Trifluridine and tipiracil and IV administrations.
Overdosage
The highest dose of trifluridine and tipiracil administered in clinical studies was 180 mg/m2 per day. There is no known antidote for trifluridine and tipiracil overdosage.
Pharmacology
Mechanism of Action
Trifluridine and tipiracil consists of a thymidine-based nucleoside analog, trifluridine, and the thymidine phosphorylase inhibitor, tipiracil, at a molar ratio 1:0.5 (weight ratio, 1:0.471). Inclusion of tipiracil increases trifluridine exposure by inhibiting its metabolism by thymidine phosphorylase.
Following uptake into cancer cells, trifluridine is incorporated into DNA, interferes with DNA synthesis and inhibits cell proliferation. Trifluridine/tipiracil demonstrated anti-tumor activity against KRAS wild-type and mutant human colorectal cancer xenografts in mice.
Structure
Trifluridine and tipiracil contains trifluridine and tipiracil hydrochloride at a molar ratio of 1:0.5.
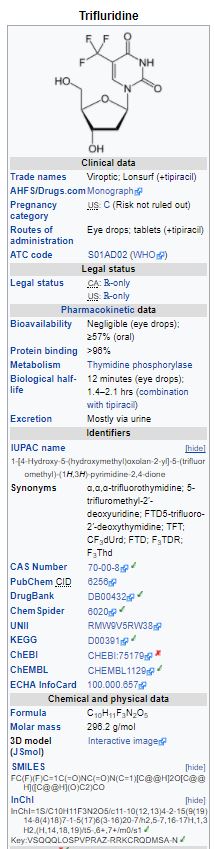
Trifluridine: Trifluridine, an antineoplastic thymidine-based nucleoside analogue, is described chemically as 2’-deoxy-5-(trifluoromethyl) uridine, and has the following structural formula:
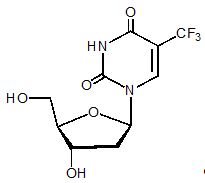
Trifluridine has a molecular formula C10H11F3N2O5 and a molecular weight of 296.20. Trifluridine is a white crystalline powder, soluble in water, ethanol, 0.01 mol/L hydrochloric acid, 0.01 mol/L sodium hydroxide solution; freely soluble in methanol, acetone; sparingly soluble in 2-Propanol, acetonitrile; slightly soluble in diethyl ether; and very slightly soluble in isopropyl ether.
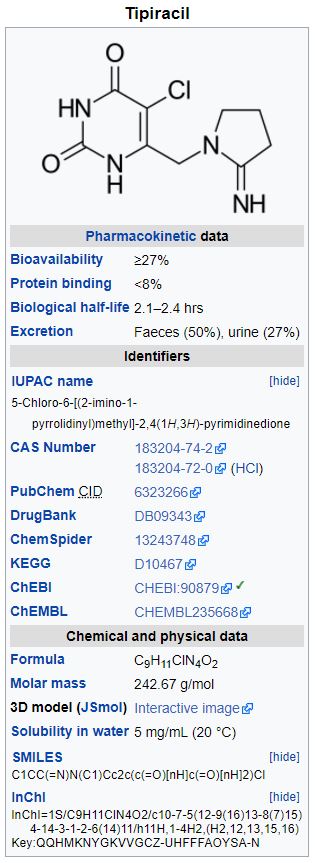
Tipiracil hydrochloride: Tipiracil hydrochloride, a thymidine phosphorylase inhibitor, is described chemically as 5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]pyrimidine-2,4-(1H,3H)-dione monohydrochloride or 2,4(1H,3H)-Pyrimidinedione, 5-chloro-6-[(2-imino-1-pyrrolidinyl)methyl]-, hydrochloride (1:1), and has the following structural formula:
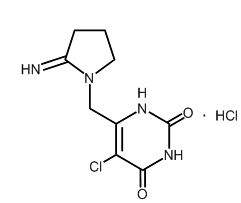
Tipiracil hydrochloride has a molecular formula C9H11ClN4O2•HCl and a molecular weight of 279.12. Tipiracil hydrochloride is a white crystalline powder, soluble in water, 0.01 mol/L hydrochloric acid, and 0.01 mol/L sodium hydroxide; slightly soluble in methanol; very slightly soluble in ethanol; and practically insoluble in acetonitrile, 2-Propanol, acetone, diisopropyl ether, and diethyl ether.
Pharmacodynamics
Cardiac Electrophysiology
Trifluridine and tipiracil administered to 42 patients with advanced solid tumors at the recommended dosage regimen had no large effect (i.e. > 20 ms) in the mean QTc interval when compared to placebo and no evident exposure-QT relationship was identified. Two of 42 patients (4.8%) had QTc greater than 500 msec and 1 of 42 patients (2.4%) had a QTc increase from baseline greater than 60 msec.
Pharmacokinetics
After twice daily dosing of trifluridine and tipiracil, systemic exposure (area under the concentration curve, AUC) of trifluridine increased more than dose-proportionally over the dose range of 15 to 35 mg/m2. After administration of trifluridine and tipiracil 35 mg/m2 twice daily, the mean elimination half-life (t½) of trifluridine was 1.4 hours and of tipiracil was 2.1 hours after a single dose. The mean elimination half-life at steady state of trifluridine was 2.1 hours and of tipiracil was 2.4 hours.
The accumulation of trifluridine was 3-fold for AUC0-last and 2-fold for peak plasma concentration (Cmax) at steady state while no accumulation was observed for tipiracil.
Administration of a single dose of trifluridine and tipiracil containing tipiracil and trifluridine 35 mg/m2 increased the mean AUC0-last of trifluridine by 37-fold and Cmax by 22-fold with reduced variability compared to trifluridine 35 mg/m2 alone.
Absorption
Following a single oral administration of trifluridine and tipiracil at 35 mg/m2 in patients with cancer, the mean time to peak plasma concentration (Tmax) of trifluridine was around 2 hours.
A standardized high-fat, high-calorie meal decreased trifluridine Cmax, tipiracil Cmax and AUC by approximately 40%, but did not change trifluridine AUC compared to those in a fasting state in patients with cancer following administration of a single dose of trifluridine and tipiracil 35 mg/m2. It is recommended to take trifluridine and tipiracil within 1 hour after completion of the morning and evening meals based on the observed correlation between the increase in the Cmax of trifluridine and the decrease in neutrophil counts.
Distribution
Trifluridine mainly binds to human serum albumin. The in vitro protein binding of trifluridine in human plasma is greater than 96%, independent of drug concentration and presence of tipiracil. Plasma protein binding of tipiracil is below 8%.
Elimination
Metabolism: Trifluridine and tipiracil are not metabolized by cytochrome P450 (CYP) enzymes. Trifluridine is mainly eliminated by metabolism via thymidine phosphorylase to form an inactive metabolite, 5-(trifluoromethyl) uracil (FTY). No other major metabolites were detected in plasma or urine.
Excretion: After single oral administration of trifluridine and tipiracil (60 mg) with [14C]-trifluridine, the total cumulative excretion of radioactivity was 60% of the administered dose. The majority of recovered radioactivity was eliminated into urine (55% of the dose) as FTY and trifluridine glucuronide isomers within 24 hours, and the excretion into feces and expired air was less than 3% for both. The unchanged trifluridine was less than 3% of administered dose recovered in the urine and feces.
After single oral administration of trifluridine and tipiracil (60 mg) with [14C]-tipiracil hydrochloride, recovered radioactivity was 77% of the dose, which consisted of 27% urinary excretion and 50% fecal excretion. Tipiracil was the major component and 6-HMU was the major metabolite in urine, and feces.
Specific Populations
Age, Sex, and Race: Based on the population pharmacokinetic analysis, there is no clinically relevant effect of age, sex, or race (White or Asian) on the pharmacokinetics of trifluridine or tipiracil.
Renal Impairment: In Study 1, the estimated mean AUC of trifluridine at steady state was 31% higher in patients with mild renal impairment (CrCl = 60 to 89 mL/min, n= 38) and 43% higher in patients with moderate renal impairment (CrCl = 30 to 59 mL/min, n= 16) than that in patient with normal renal function (CrCl ≥ 90 mL/min, n= 84) based on the population pharmacokinetic analysis. The estimated mean AUC of tipiracil was 34% higher in patients with mild renal impairment and 65% higher in patients with moderate renal impairment than that in patients with normal renal function. The pharmacokinetics of trifluridine and tipiracil have not been studied in patients with severe renal impairment (CrCl < 30 mL/min) or end-stage renal disease.
Hepatic Impairment: In a pharmacokinetic trial of patients with hepatic impairment, no clinically important differences in the mean exposures of trifluridine and tipiracil were observed between patients with mild hepatic impairment (total bilirubin less than or equal to the ULN and AST greater than ULN or total bilirubin less than 1 to 1.5 times ULN and any AST) to moderate hepatic impairment (total bilirubin greater than 1.5 to 3 times ULN and any AST) and patients with normal hepatic function (total bilirubin and AST less than or equal to the ULN). Five of 6 patients with moderate hepatic impairment experienced Grade 3 or 4 increased bilirubin levels and patients with severe hepatic impairment were not studied.
Drug Interaction Studies:
Trifluridine is a substrate of thymidine phosphorylase, and is not metabolized by cytochrome P450 (CYP) enzyme. Tipiracil is not metabolized in either human liver or hepatocytes.
In vitro studies indicated that trifluridine, tipiracil, and FTY did not inhibit the CYP enzymes and had no inductive effect on CYP1A2, CYP2B6, or CYP3A4/5.
In vitro studies indicated that trifluridine was not an inhibitor of or substrate for human uptake and efflux transporters.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies evaluating the carcinogenic potential of trifluridine/tipiracil in animals have been performed. Trifluridine/tipiracil was genotoxic in a reverse mutation test in bacteria, a chromosomal aberration test in mammalian-cultured cells, and a micronucleus test in mice.
Animal studies did not indicate an effect of trifluridine/tipiracil on male fertility in rats. Dose-related increases in the corpus luteum count and implanted embryo count were observed, but female fertility was not affected.
Clinical Studies
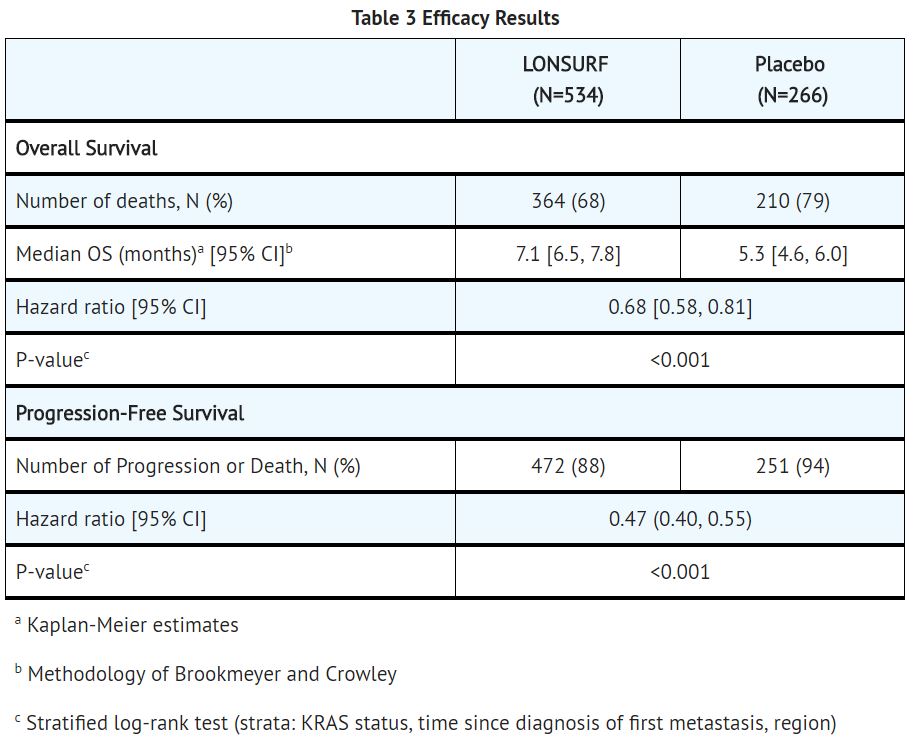
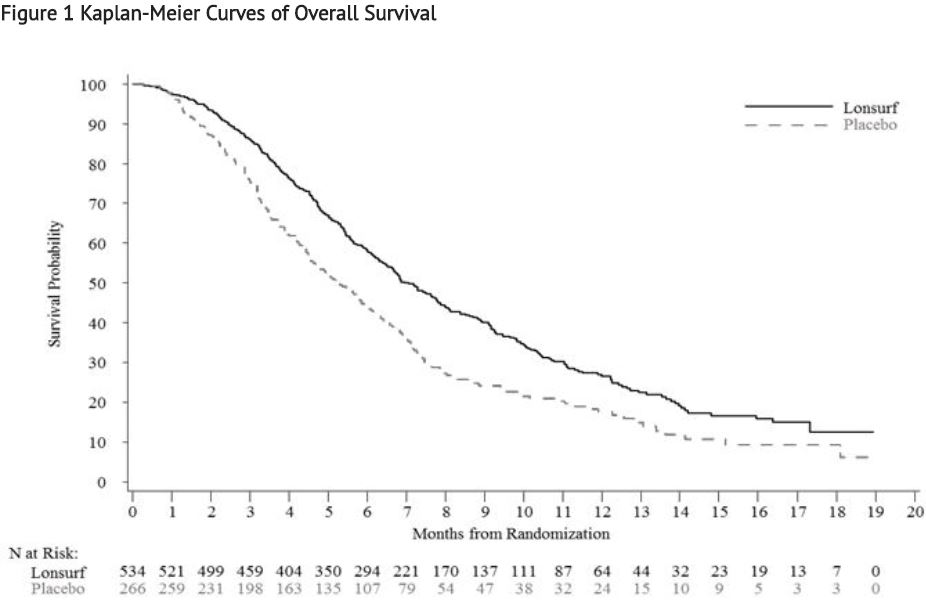
Study 1: The clinical efficacy and safety of trifluridine and tipiracil were evaluated in an international, randomized, double-blind, placebo-controlled study conducted in patients with previously treated metastatic colorectal cancer (CRC).
A total of 800 patients were randomized 2:1 to receive trifluridine and tipiracil (N=534) plus best supportive care (BSC) or matching placebo (N=266) plus BSC. Randomization was stratified by KRAS status (wild-type vs. mutant), time since diagnosis of first metastasis (<18 months vs. ≥ 18 months), and region (Japan vs. US, Europe and Australia). Key eligibility criteria included prior treatment with at least 2 lines of standard chemotherapy for metastatic CRC, ECOG 0-1, absence of brain metastasis, and absence of ascites requiring drainage in the past four weeks. Patients received 35 mg/m2 trifluridine and tipiracil or matching placebo orally twice daily after meals on Days 1 - 5 and 8 – 12 of each 28-day cycle until disease progression or unacceptable toxicity. The major efficacy outcome measure was overall survival (OS) and an additional efficacy outcome measure was progression-free survival (PFS). The median age was 63 years, 61% were male, 58% and 35% were White and Asian respectively, and all patients had baseline ECOG Performance Status (PS) of 0 or 1. The primary site of disease was colon (62%) or rectum (38%). KRAS status was wild-type (49%) or mutant (51%) at study entry. All patients received prior treatment with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy. All but one patient received bevacizumab, and all but two patients with KRAS wild-type tumors received panitumumab or cetuximab.
A statistically significant improvement in overall survival and progression-free survival were demonstrated in patients in the trifluridine and tipiracil plus BSC arm compared to those who received placebo plus BSC.
How Supplied
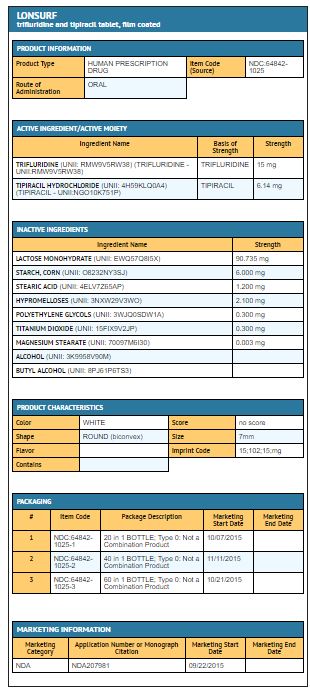
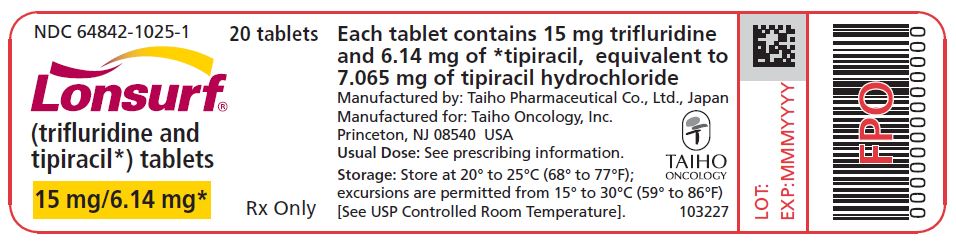
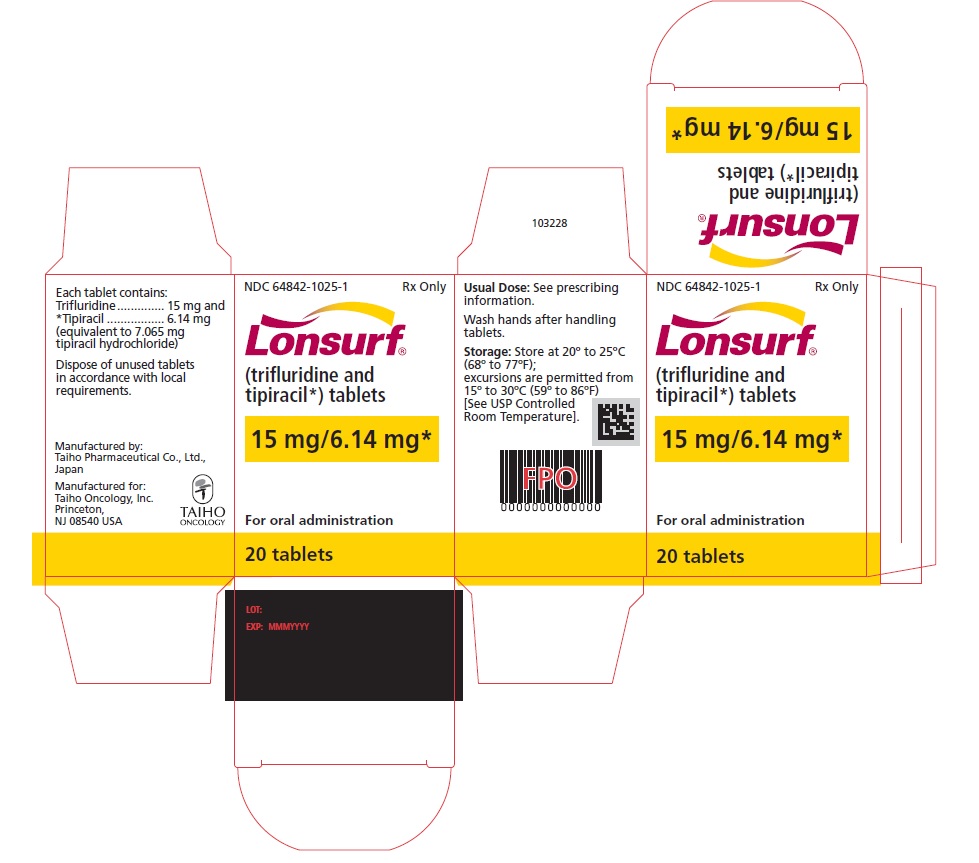
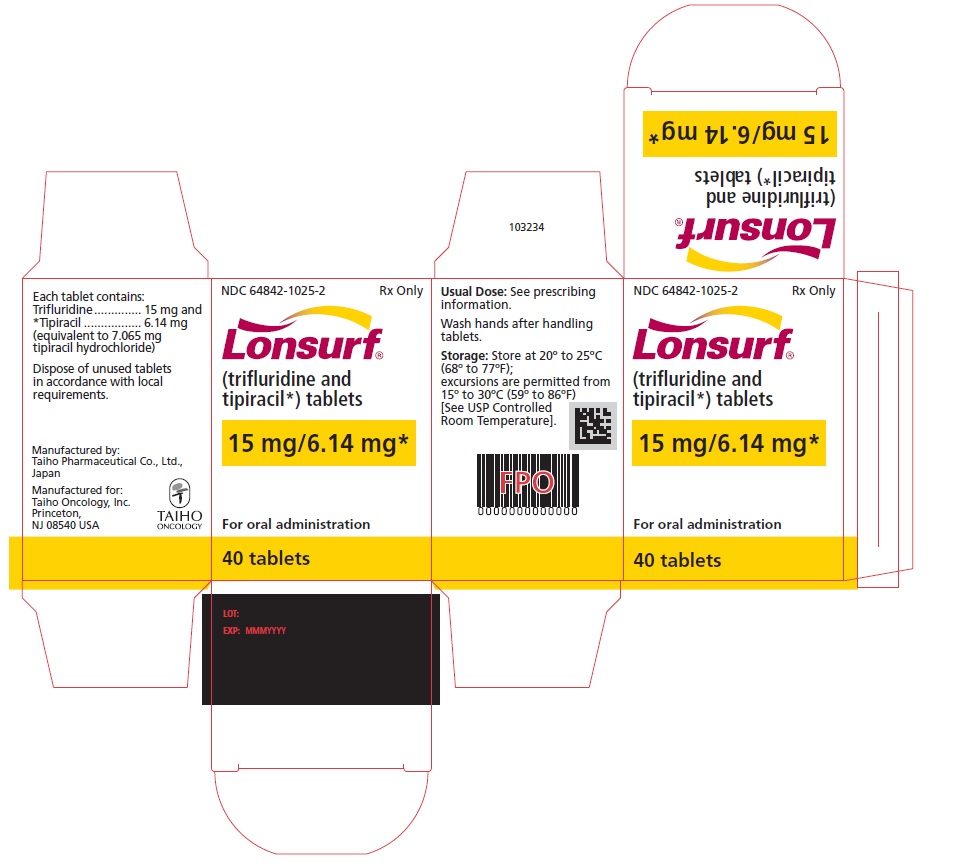
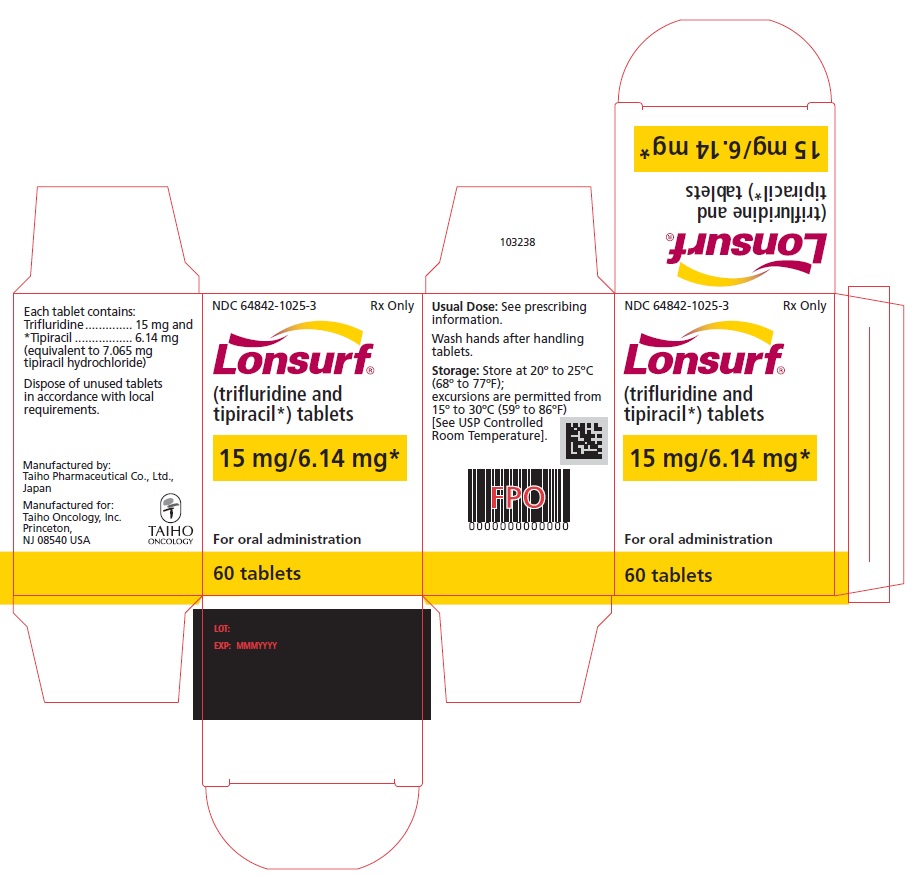
Trifluridine and tipiracil 15 mg/6.14 mg tablets are supplied as white, biconvex, round, film-coated tablet, imprinted with ‘15’ on one side, and ‘102’ and ’15 mg’ on the other side, in gray ink. The tablets are packaged in HDPE bottles with child resistant closures in the following presentations:
- 20 count: NDC 64842-1025-1
- 40 count: NDC 64842-1025-2
- 60 count: NDC 64842-1025-3
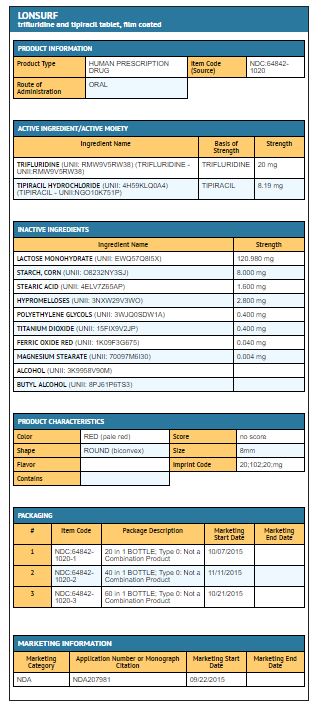
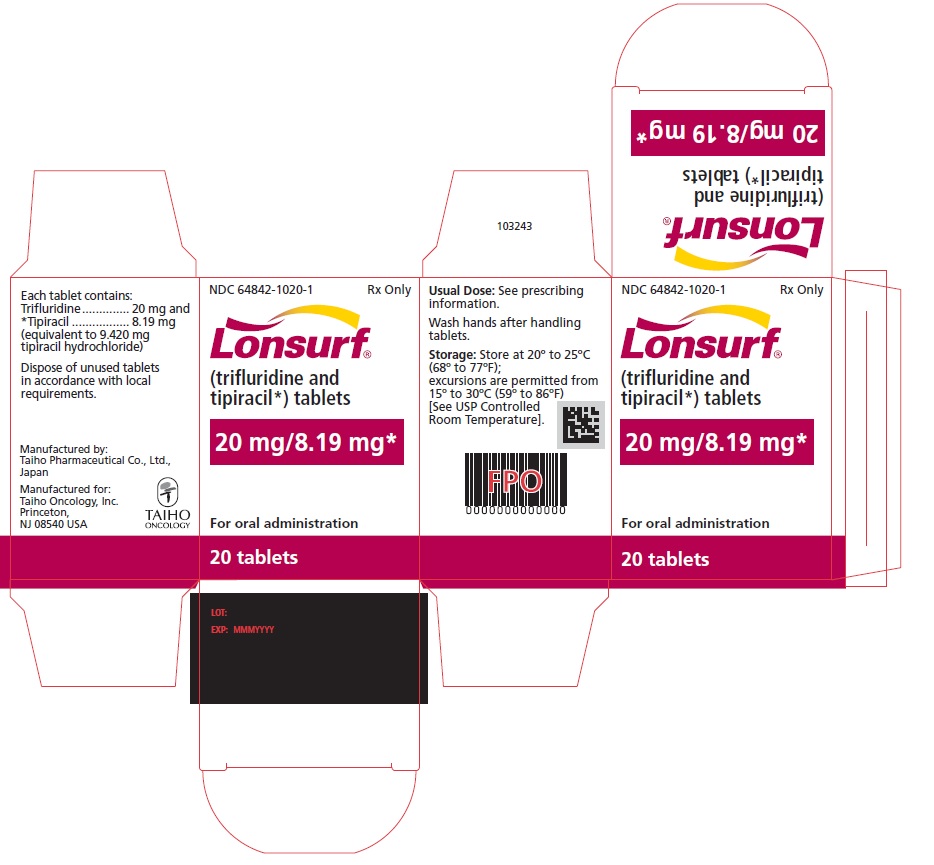
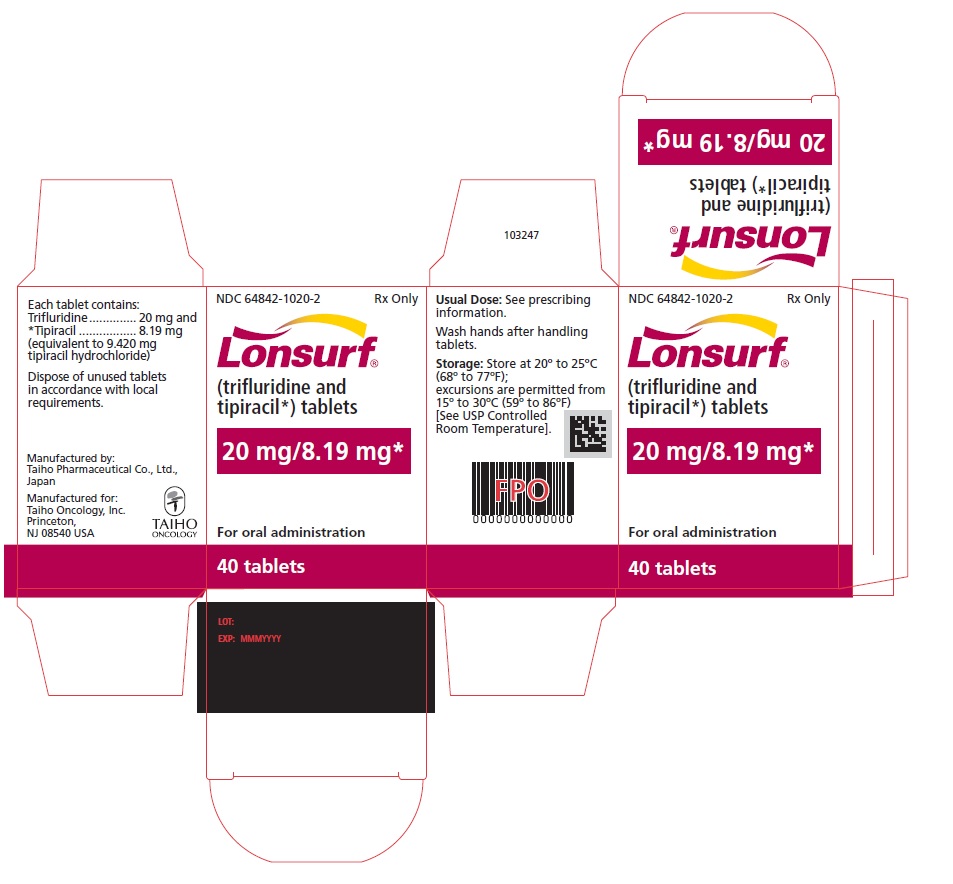
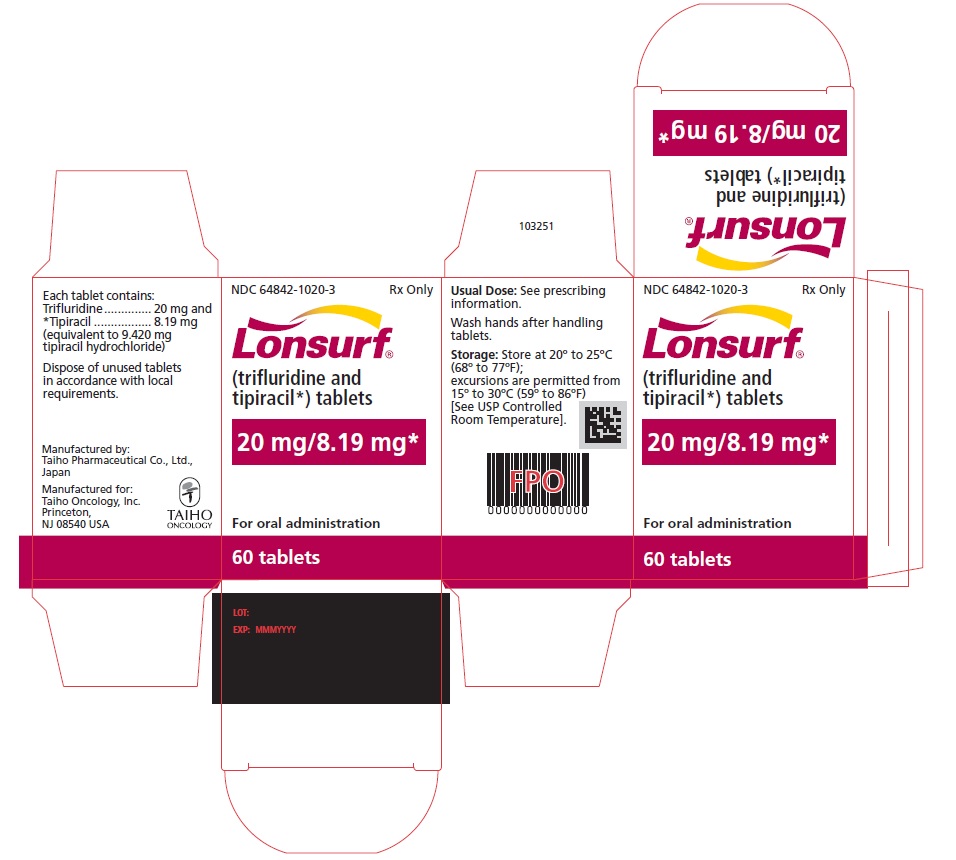
Trifluridine and tipiracil 20 mg/8.19 mg tablets are supplied as pale red, biconvex, round, film-coated tablet, imprinted with ‘20’ on one side, and ‘102’ and ‘20 mg’ on the other side, in gray ink. The tablets are packaged in HDPE bottles with child resistant closures in the following presentations:
- 20 count: NDC 64842-1020-1
- 40 count: NDC 64842-1020-2
- 60 count: NDC 64842-1020-3
Storage
Store at 20°C to 25°C (68°F to 77°F); excursions are permitted from 15°C to 30°C (59°F to 86°F). Trifluridine and tipiracil is a cytotoxic drug. Follow applicable special handling and disposal procedures. If stored outside of original bottle, discard after 30 days.
Images
Drug Images
{{#ask: Page Name::Trifluridine and tipiracil |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel





{{#ask: Label Page::Trifluridine and tipiracil |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Advise the patient to read the FDA-Approved Patient Labeling.
- Severe Myelosuppression
- Advise the patient to immediately contact their healthcare provider if they experience signs or symptoms of infection and advise patients to keep all appointments for blood tests.
- Gastrointestinal toxicity
- Advise patients to contact their healthcare provider for severe or persistent nausea, vomiting, diarrhea, or abdominal pain.
- Administration Instructions
- Advise the patient that trifluridine and tipiracil is available in two strengths and they may receive both strength tablets to provide the prescribed dose. Advise the patient of the importance of reading prescription labels carefully and taking the appropriate number of tablets.
- Advise the patient to take trifluridine and tipiracil within 1 hour after eating their morning and evening meals.
- Advise the patient that anyone else who handles their medication should wear gloves.
- Embryo-Fetal Toxicity
- Advise pregnant women of the potential risk to the fetus.
- Advise females of reproductive potential to use effective contraception during treatment with trifluridine and tipiracil.
- Lactation
- Advise women not to breastfeed during treatment with trifluridine and tipiracil and for one day following the final dose.
Precautions with Alcohol
Alcohol-Trifluridine and tipiracil interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
LONSURF
Look-Alike Drug Names
There is limited information regarding Trifluridine and tipiracil Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.