Transjugular intrahepatic portosystemic shunt: Difference between revisions
| Line 142: | Line 142: | ||
|- | |- | ||
| | | | ||
* | * Encephalopathy (15%) | ||
* Bleeding (hemoperitoneum; 3%) | * Bleeding (hemoperitoneum; 3%) | ||
* Death (1%) | |||
* Trauma to liver and heart | * Trauma to liver and heart | ||
* Stent infection | * Stent infection | ||
| Line 149: | Line 150: | ||
* Heart failure | * Heart failure | ||
* Stent thrombosis | * Stent thrombosis | ||
* Stent malposition | * Stent malposition | ||
* Nephropathy | * Nephropathy | ||
* | * Inability to place stent | ||
| | | | ||
* Stent thrombosis | * Stent thrombosis | ||
| Line 161: | Line 161: | ||
* Radiation injury | * Radiation injury | ||
*Umbilical hernia<ref name="pmid17825762">{{cite journal |author=Mallavarapu RK, Grimsley EW |title=Incarcerated umbilical hernia after transjugular intrahepatic portosystemic shunt procedure for refractory ascites |journal=Clin. Gastroenterol. Hepatol. |volume=5 |issue=9 |pages=A26 |year=2007 |pmid=17825762 |doi=10.1016/j.cgh.2007.07.018}}</ref> | *Umbilical hernia<ref name="pmid17825762">{{cite journal |author=Mallavarapu RK, Grimsley EW |title=Incarcerated umbilical hernia after transjugular intrahepatic portosystemic shunt procedure for refractory ascites |journal=Clin. Gastroenterol. Hepatol. |volume=5 |issue=9 |pages=A26 |year=2007 |pmid=17825762 |doi=10.1016/j.cgh.2007.07.018}}</ref> | ||
*In-stent stenosis | *In-stent stenosis | ||
|} | |} | ||
Encephalopathy (immediate complication) may be amenable to medical therapy<sup> </sup>; if it is severe, the shunt may have to be narrowed or embolized. As for In-stent stenosis a greater than 50% stenosis is seen in 25% of TIPS cases; this can be addressed with angioplasty. | |||
Technical complications. | |||
Technical complications. | |||
Unfortunately, prospective studies investigating the technical complications are not available. Perforation of the liver capsule without or with intraperitoneal hemorrhage has been described in 33 and 1–2% of the procedures, respectively. However, in centers using sonography during the puncture process, these complications are almost abolished. The same is true for clinically significant hemobilia or hemolysis, complications which have been seen more frequently at the beginning of the TIPS era. With the use of modern stents, stent misplacement or migration is also very rare. Frequencies of 20% proximal or distal displacement given in a recent review [20] are, in our experience, unusual. In particular, the Viatorr stent is designed to be placed with great accuracy and misplacement is almost impossible | Unfortunately, prospective studies investigating the technical complications are not available. Perforation of the liver capsule without or with intraperitoneal hemorrhage has been described in 33 and 1–2% of the procedures, respectively. However, in centers using sonography during the puncture process, these complications are almost abolished. The same is true for clinically significant hemobilia or hemolysis, complications which have been seen more frequently at the beginning of the TIPS era. With the use of modern stents, stent misplacement or migration is also very rare. Frequencies of 20% proximal or distal displacement given in a recent review [20] are, in our experience, unusual. In particular, the Viatorr stent is designed to be placed with great accuracy and misplacement is almost impossible | ||
Revision as of 01:07, 12 February 2018
| Transjugular intrahepatic portosystemic shunt | |
 | |
|---|---|
| TIPS Procedure |
| https://www.youtube.com/watch?v=O2u4_hF3234%7C350}} |
|
Transjugular intrahepatic portosystemic shunt Microchapters |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Umar Ahmad, M.D.[2]
Overview
A transjugular intrahepatic portosystemic shunt, also TIPS, is an artificial channel in the liver from the portal vein to a hepatic vein (for blood). It is created endovascularly (via the blood vessels) by physicians via the jugular vein.
It is used to treat portal hypertension (which often is due to scarring of the liver (liver cirrhosis) which frequently leads to intestinal bleeding (esophageal varices) or the buildup of fluid within the abdomen (ascites).
History
- In 1969, Transjugular intrahepatic portosystemic shunt (TIPS) was first described by Joseph Rösch et al in dogs. Patency lasted 2 weeks.[1]
- In 1982, Colapinto and Gordon were the first to use this procedure in a human clinical trial but at the time the results were not encouraging and had a high mortality rate in which 9 people died within the month.[2][3]
- In the Mid-1980's, J. C. Palmaz achieved long-term patency with the introduction of expandable metallic stents and was thus approved by the local ethics committee in 1987. The procedure took an average of 8 hours and the long-term patency lasted as long as 9 months.[4]
- In 1990, Jean Marc Perarnau helped improved the puncture techinque which led to a reduction in procedure time.The procedure took an average of 1 to 2 hours.[5]
- In 1995, Nishimine K et al experimented on expanded polytetrafluoroethylene-covered (PTFE) stent grafts that last longer and are currently used for TIPS creation by most interventionalists.[6]
Indications
Accepted indications for TIPS are due to the data available on controlled-trials. The following are accepted indications:
- Uncontrolled variceal hemorrhage from esophageal, gastric, and intestinal varices that do not respond to endoscopic and medical management
- Refractory ascites
- Hepatic pleural effusion (hydrothorax)
Controversial indications for TIPS are due to the data available in uncontrolled-trials. The following are controversial indications:
- Bridge to transplantation and retransplantation
- Budd-Chiari syndrome
- Hepatic Hydrothorax
- Hepatorenal syndrome (HRS)[7]
- Veno-occlusive disease (VOD)
Contraindications
Absolute contraindications for TIPS include the following:
- Severe encephalopathy
- Severe and progressive liver failure (on the basis of the Child-Pugh score; scores A and B have a better outcome than score C). Rapid increase in bilirubin concentration requires immediate TIPS occlusion to prevent death.
- Severe right-heart failure
- Polycystic liver disease
Relative contraindications for TIPS include the following:
- Pulmonary hypertension
- Portal and hepatic vein thrombosis
- Bilirubin >3 mg/dl
- Hepatopulmonary syndrome
- MELD score above 18
- Active infection
- Tumor within the expected path of the shunt
- Cardiac Failure. Pre-TIPS and post-TIPS E/A ratio measurements.
Preprocedure
Patient Prep
Preparing before for the procedure includes the following important steps:
- Obtain informed consent
- Review preprocedural vascular ultrasound studies or computed tomography (CT) scans of the abdomen to confirm the patency of the portal vein and assess for anatomic limitations.
- Confirm that the patient has no contrast allergy
- Check for a platelet count higher than 50,000/μL
- Relatively normal international normalized ratio (INR)
- Consider broad-spectrum antibiotic prophylaxis, preferably Ceftriaxone.[8]
- Position the patient supine, with the neck turned away from the side of vein puncture. Avoid pillows unless they are needed.
- Determine the Model for End-stage Liver Disease (MELD) score; this helps predict TIPS mortality, which is higher with a MELD score of 18 or above. Addition of sodium assessment to the MELD score may further enhance prediction of TIPS mortality.
- Anesthesia administration. General anesthesia is usually required for pediatric patients and is preferred in many institutions for adults as well. Procedural sedation may be used, depending on local practices. Sedation may vary depending on continent for example, in Europe, Midazolam, Piritamide, and Propofol are preferentially given while in the U.S general anesthesia with endotracheal intubation is preferred. Midazolam with Fentanyl Citrate is a reasonable combination for achieving procedural sedation. Local anesthesia is achieved with approximately 5 mL of Lidocaine 1% at the jugular puncture site.
- If the patient has ascites with significant volume, perform paracentesis first.
Equipment
Equipment used for transjugular intrahepatic porto-systemic shunt (TIPS) creation includes the following:
- Fluoroscopy
- Pressure transducer
- Basic angiography set
- Medical CO 2 and its kit
- Sheath, 5 French, and curved catheter
- ultrasound machine with a linear-array probe
- Angioplasty balloons, typically 8 mm × 40 mm
- Chlorhexidine or povidone-iodine solution for skin disinfection
- Bare stents may be used, if needed, to extend to the right atrium
- Heparinized saline (1000-2000 U heparin in 1000 mL of 0.9% NaCl)
- Guide wires - 0.035-in. Terumo glidewire and exchange-length 0.035-in. Amplatz wire.
- TIPS kit (Cook Medical, Bloomington, IN; see the first image below) - Sheath, 10 French, 40 cm; guide catheter, 51 cm, with metal stiffener; portal venous access needle, 60 cm
- Covered stent - Gore Viatorr (WL Gore, Flagstaff, AZ) or another brand, such as Wallgraft (Boston Scientific, Natick, MA; the advantage of the Gore Viatorr is the design, which consists of a distal 2-cm-long unlined segment that is deployed in the portal vein and therefore gives better anchorage without obstructing the flow and allows for nutrient portal perfusion
Procedure
Transjugular intrahepatic portosystemic shunt (TIPS) creation proceeds as follows:
Procedure
- Positioning- The patient will be positioned on their back.
- Monitoring- The patient will then be connected to monitors that track your heart rate, blood pressure and pulse during the procedure.
- Line placement- A nurse or technologist will insert an intravenous (IV) line into a vein in the patients hand or arm so that sedative medication can be given intravenously. Moderate sedation may be used. As an alternative, you may receive general anesthesia.
- Sterilization- The area of the patient's body where the catheter is to be inserted will be sterilized and covered with a surgical drape. Clean the skin on the neck with chlorhexidine or povidone-iodine solution.
- Anesthetic- Numbing the area just above your right collarbone with a local anesthetic.
- Incision for catheter- Make a small (≤1 cm) horizontal skin incision.
- Identification of vessel- Using ultrasonographic guidance and a micropuncture or an 18-gauge access needle, puncture the anterior wall of the vein, and enter the vein. Aspirate venous blood to confirm the needle position..
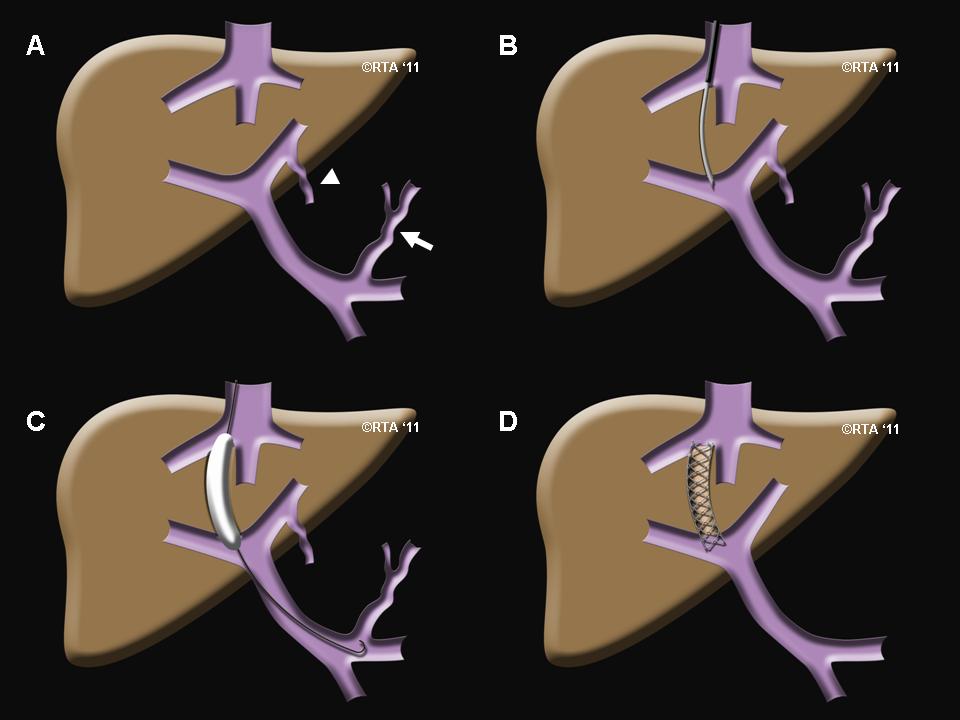
- Guidance of catheter- Using real time x-ray guidance, advance the 0.035-in. guide wire, and insert the accompanying 5-French sheath over the wire. Use a curved catheter and Terumo hydrophilic wire to access the right hepatic vein. Wedge the catheter in the hepatic vein. Obtain wedged hepatic and inferior vena cava (IVC)/right atrial pressure measurements, and calculate the gradient to confirm the diagnosis of portal hypertension, and also to determine the severity of the condition. To help plan for the placement of the TIPS stent, a contrast material will be injected in the hepatic vein to identify the portal venous system. Access is then gained from the hepatic vein into the portal system using a TIPS needle (a special long needle extending from the neck into the liver). Perform portal angiography (if available), using medical CO2. Because the catheter is wedged, an indirect portogram along with a hepatic venogram can be obtained. Use the images to confirm the patency of both veins. Use the image as a fluoroscopic fade/roadmap, or mark the portal vein and hepatic veins on screen. Either way, lock the table in position.
- Tract formation via Dilation- Insert an Amplatz wire, and then exchange the Cobra-2 catheter and 5-French sheath for the 10-French sheath with dilator. Remove the dilator, and introduce the inner sheath, loaded together with the metal stiffener (stent). Insert the system up to 1 cm from the point of intersection of the two veins. Remove the wire, and insert the catheter with the needle. Turn the system, using the metal arrow on the stiffener anteriorly (on the assumption of placement in the right hepatic vein), and advance the needle with the catheter in an anteroinferior direction parallel to the spine, aiming for the portal vein. Start the throw approximately 2 cm from the confluence of the right hepatic vein and the IVC. The right portal vein is typically accessed 0.5-1.5 vertebral body widths lateral to the spine between T10 and T12. Remove the needle, and attach a small syringe with 1 mL of contrast to the catheter. Aspirate while gently withdrawing. A loss of resistance is felt when in the portal vein. Confirm by aspiration and then injection of contrast. Once in the portal vein, insert a Terumo Glidewire. Advance the wire into the superior mesenteric vein or the splenic vein, and advance the catheter. Obtain portal and central pressure measurements to confirm the gradient. Exchange the Terumo wire for an 180-cm Amplatz wire. Perform angioplasty of the tract (with a 6- or 8-mm balloon). Advance a 10-French sheath into the portal vein; replacing the dilator is likely to be helpful. Exchange the catheter for a calibrated pigtail catheter. Perform a double-flush angiogram via both the pigtail and the side arm of the sheath (with the inner metal stiffener removed and sheath pulled back into the hepatic vein) to obtain images of hepatic and portal veins. Use these images to calculate the length of stent required. Measure from the portal vein to the confluence of the hepatic vein and the IVC. To correctly size a Viatorr stent, add 1-2 cm to the measured length. Reinsert the Amplatz wire, and advance the sheath back into the portal vein.
- Placement of stent- Remove the pigtail catheter then insert the stent over the wire into position; be careful to keep the Viatorr stent confined by the packaging sheath until it is fully introduced into the 10-French sheath, and do not advance the stent beyond the sheath tip in the portal vein. Sheath and stent should extend approximately 3 cm into the portal vein. Withdraw the outer TIPS sheath, and then deploy the stent as per its prescribed mechanism. With the Viatorr, an upstream 2-cm-long uncovered segment is deployed by withdrawing the sheath. Positioning may be fine-tuned at this point to achieve the goal of placing the uncovered portion in the portal vein and the covered portion in the tract/hepatic vein. Undersizing the initial tract angioplasty may help give a tactile sense of the junction of the portal vein and the tract. Once the stent is in the correct position, the balloon is inflated, expanding the stent into place. Perform a portogram to assess flow through stent and any waisting. Measure pressures to ensure a typical goal portohepatic gradient of less than 12 mm Hg (for confirmation of a reduction in portal hypertension). If necessary, dilate the stent with an 8-mm balloon. If the stent does not reach the confluence of the hepatic vein and the IVC, it may be extended with an additional uncovered stent. The balloon is then deflated and removed along with the catheter.
- Closing up- Pressure will be applied to prevent any bleeding and the opening in the skin is covered with a bandage. No sutures are needed.
- Observation- You will be admitted to the hospital following your procedure, where you will be closely observed.
- Completion- This procedure is usually completed in an hour or two but may take up to several hours depending on the complexity of the condition and vascular anatomy.
You will be positioned on your back, connecting you to monitors where your blood pressure, pulse and heart rate will all be traced while the TIPS procedure is being conducted. The specific area of your body where the catheter will be inserted will be shaved, sterilized and covered with a surgical drape preparing you for the start of the procedure. The upper part of your right collarbone will be numbed with a local anaesthetic before a small cut is made in the skin at the site. With the use of ultrasound, the specialist will identify the patient’s internal jugular vein and guide a catheter into the vessel towards the liver and into the hepatic vein. To confirm the diagnosis of portal hypertension and its severity, pressure is also measured within the right side of the heart and in the hepatic vein. A contrast material will be injected into the hepatic vein to help place the TIPS stent and to identify the portal venous system. An entry is then gained from the hepatic vein into the portal system using a TIPS needle. The stent will be placed under the fluoroscopy extending from the portal vein into the hepatic vein. Once the stent is properly placed, the balloon is inflated, expanding the stent into place. The skin will then be covered with a bandage leaving you with no need for sutures. Close follow up observations are needed after your operation which will be conducted within the hospital.
Postprocedure
Monitoring and Follow-up
The high frequency of shunt stenosis warrants close surveillance with Doppler ultrasonography or portography. Patients undergo a baseline Doppler study within 24 hours of the procedure to document functional parameters, including the direction of portal vein flow and flow velocities throughout the shunt and within the hepatic vein. Although TIPS venography with direct portal and right atrial pressure measurements is the criterion standard for stent assessment, high sensitivity and specificity for shunt function has been reported with certain Doppler criteria, as follows:
- Absent flow
- Low peak shunt velocity (<50 to 90 cm/s)
- High peak shunt velocity (190 cm/s)
- Low mean PV velocity (<30 cm/s)
- Return of antegrade flow in the intrahepatic PVs
- Significant change in shunt velocity (>50 cm/s) as compared with the immediate postprocedural result
In the acute phase, the stent can thrombose. To treat this, the stent can be lysed, or mechanical thrombectomy can be performed. If later in-stent stenosis occurs, perform angioplasty or insert another stent, as required.
Postprocedural follow-up for TIPS placement is important to ensure patency. Post-TIPS Doppler ultrasonography may be performed at 24 hours, 3 months, 6 months, 12 months, and annually thereafter.
Doppler ultrasound is the most valuable means to estimate shunt function. The parameters which should be evaluated before and after TIPS implantation are summarized in Table 1. In general, the pre-procedural low-flow velocity in the portal vein (Vmax: 10–20 cm/sec) increases by TIPS by a factor of 2–4 to 40–60 cm/sec [36–41]. A post-TIPS portal vein flow velocity of less than 30 cm/sec suggests shunt insufficiency. The flow velocity in the stent is expected to be between 80 and 160 cm/sec shortly after TIPS. Values below 60 or above 180 cm/sec indicate shunt insufficiency. In particular, any value lower than 40 or higher than 200 cm/sec clearly indicates shunt malfunction [41]. It should be emphasized that measurements in the stent-shunt or in the draining hepatic vein are only reliable in cases of simple stenoses (Figs. 2 and 3). In cases with a complex structure of the intimal proliferation in the stent or in the draining hepatic vein, the measurements are not reliable and normal values cannot exclude stenosis (Figs. 2 and 4). Therefore, in case of normal values within the stent, the findings in the portal vein define whether shunt function is sufficient or not. In addition, a change in flow direction of the intrahepatic portal branches from retrograde shortly after TIPS to prograde may also be a good qualitative indicator of shunt malfunction [37]. If simple stenosis is seen, the Bernoulli equation (Δp = 4 v2) can be applied to calculate the pressure gradient Δp (in mmHg) across the stenosis from the flow velocity measured in the stenosis (Vmax in m/sec). Accordingly, a flow velocity (Vmax) of 180 cm/sec (1.8 m/sec) indicates a pressure gradient across the stenosis of 13 mmHg. It could be demonstrated that calculated gradients using the Bernoulli equation closely correlate with gradients determined by catheter measurement (r = 0.84) [41].The early post-procedural setting consists of monitoring of the blood pressure, haemoglobin/hematocrit and maybe urine volume during 24 hours. With few exceptions, intensive care is not necessary. In general, in patients with variceal bleeding, ß-blockers are withdrawn and in patients with refractory ascites, diuretic medication is reduced by half. A Duplex-sonographic examination is performed before patient’s discharge.
Outcome
The technical success of TIPS placement is related to the experience and skill of the interventional radiologist. Data from three large centers (University of California, San Francisco; University of Pennsylvania; and the Freiberg group) demonstrated technical success rates of more than 90%.
Successful TIPS placement results in a portosystemic gradient of less than 12 mm Hg and immediate control of variceal-related bleeding. A target portosystemic gradient of 12 mm Hg is used as varices tend not to bleed when the gradient is less than 12 mm Hg. When technical failure occurs, it is usually due to an anatomic situation that prevents acceptable portal venous puncture. Significant reduction in ascites usually occurs within 1 month of the procedure, and this is estimated to occur in 50-90% of cases. [[null 7], [null 8], [null 9], [null 10]]
Late stenosis and occlusion are usually related to pseudointimal hyperplasia within the stent or, more commonly, intimal hyperplasia within the hepatic vein. In most cases, the stenotic stent can be crossed with a guide wire and recanalized with balloon dilation (see the image below) or repeat stent placement to improve long-term patency rates. Primary patency after TIPS placement has been reported to be 66% and 42% after 1 and 2 years. Primary-assisted patency rates at 1 and 2 years are reported to be 83% and 79%, respectively, and secondary patency rates at 1 and 2 years are reported to be 96% and 90%. null 8
Balloon angioplasty used to treat hyperplasia.
Reported figures for 30-day mortality vary among centers, and nearly all centers report few or no deaths directly related to the procedure itself. Early mortality has been shown to be related to the Acute Physiology and Chronic Health Evaluation (APACHE) II score. Patients with severe systemic disease with an APACHE II score higher than 20 have a greater risk for early mortality, compared with others.
Patients with active bleeding during the procedure also have increased early mortality. The 30-day mortality is in the range of 3-30%; the variation within this range is related to the preprocedural Child classification and whether the procedure was performed on an emergency basis or an elective basis. null 11 In 1995, LaBerge et al reported that cumulative survival rates in patients with Child grades of A, B, and C, respectively, were 75%, 68%, and 49% at 1 year and 75%, 55%, and 43% at 2 years.
Complications
| Immediate complications | Delayed complications |
|---|---|
|
|
Encephalopathy (immediate complication) may be amenable to medical therapy ; if it is severe, the shunt may have to be narrowed or embolized. As for In-stent stenosis a greater than 50% stenosis is seen in 25% of TIPS cases; this can be addressed with angioplasty.
Technical complications.
Unfortunately, prospective studies investigating the technical complications are not available. Perforation of the liver capsule without or with intraperitoneal hemorrhage has been described in 33 and 1–2% of the procedures, respectively. However, in centers using sonography during the puncture process, these complications are almost abolished. The same is true for clinically significant hemobilia or hemolysis, complications which have been seen more frequently at the beginning of the TIPS era. With the use of modern stents, stent misplacement or migration is also very rare. Frequencies of 20% proximal or distal displacement given in a recent review [20] are, in our experience, unusual. In particular, the Viatorr stent is designed to be placed with great accuracy and misplacement is almost impossible
Pearls
Pearls
Always perform Doppler ultrasonography to assess the portal vein before starting the TIPS procedure to confirm that the portal vein is not thrombosed.
If the middle hepatic vein is used, remember to rotate the TIPS sheath and stiffener posteriorly because the middle portal vein lies posterior and inferior to the hepatic vein.
To confirm entry into portal vein, inject contrast material. Contrast flowing toward the right atrium indicates hepatic vein location. Static contrast likely indicates biliary system location. Contrast flowing to liver periphery can indicate location in the hepatic artery or portal vein; however, the portal radicals are larger in size.
If insertion is unsuccessful, pull the catheter back into the sheath, reinsert the needle, and try again.
When using Amplatz wires, always keep the tip of the wire under control. If the wire tip is not properly controlled, it can easily perforate the liver or the mesentery.
If covered shunt patency is difficult to assess on follow-up Doppler ultrasonography, CT may be performed.
Technical advancements in skills and stents have reduced complications and improved patency of TIPS. The major obstacle remains hepatic encephalopathy, which requires proper selection of patients and smaller shunts. In patients with acute variceal bleeding and high risk of early rebleeding, recent studies showed improved survival recommending early TIPS implantation. With respect to the prevention of rebleeding (secondary prophylaxis), TIPS remains the second-line treatment unless new studies with covered stents demonstrate its superiority over standard medical therapy. As demonstrated in 2 relevant meta-analyses, TIPS improves survival in patients with refractory ascites, justifying its earlier application. It clearly reverses the circulatory dysfunction which leads to normalization of the renal function. With a 10-year survival rate of 80%, TIPS is the preferred treatment in patients with Budd-Chiari syndrome who do not respond sufficiently to medical treatment. The meta-analysis of individual patients’ data by Salerno et al.[59] showed that TIPS patients lived significantly longer than patients treated with paracentesis. TIPS also seems to improve the estimated transplant free survival in patients with MELD scores between 10 and 20, suggesting that even patients with severe disease may benefit from TIPS.
In contrast to serial paracentesis, TIPS leads to a significant improvement of these parameters including total body nitrogen and total body protein [64–66], muscle mass, and albumin concentration [67].
Mechanism of action
A TIPS decreases the effective vascular resistance of the liver. The result is a reduced pressure drop over the liver and a decreased portal venous pressure. This, in turn, lessens the pressure on the blood vessels in the intestine so that future bleeding is less likely to occur. The reduced pressure also makes less fluid develop, although this benefit may take weeks or months to occur.
Implantation
Transjugular intrahepatic portosystemic shunts are typically placed by interventional radiologists under fluoroscopic guidance.[10] Access to the liver, as the name transjugular suggests, is gained via the jugular vein in the neck. Once access to the jugular vein is confirmed, a guidewire and introducer sheath is typically placed to facilitate the shunt's placement. This enables the interventional radiologist to gain access to the patient's liver vein (hepatic vein) by passing through the heart. The shunt is created by advancing a special needle through the sheath system to connect the hepatic vein to the large vein near the center of the liver, the portal vein. The channel for the shunt is next created by inflating an angioplasty balloon within the liver along the tract of created by the needle. The shunt is completed by placing a special mesh tube known as a stent or endograft to establish the connection between the high pressure portal vein with the lower pressure hepatic vein. After the procedure, fluoroscopic images are made to show placement and pressure measurements in the portal vein and inferior vena cava are often done.A more sophisticated and variable approach has been reported recently [72]. Two stents were released within the original stent-shunt. One of the stents is a covered stent which remains patent. The other stent is bare and short and placed besides the covered stent. The final flow volume through the covered stent can be adjusted by expansion of the bare stent. In view of the encouraging results with the hourglass-stent and a potential risk of stent migration, the need and advantage of this latter procedure seem to be questionable.
Related Chapters
Reference
- ↑ Rösch J, Hanafee WN, Snow H (1969). "Transjugular portal venography and radiologic portacaval shunt: an experimental study". Radiology. 92 (5): 1112–4. doi:10.1148/92.5.1112. PMID 5771827.
- ↑ Colapinto RF, Stronell RD, Birch SJ, Langer B, Blendis LM, Greig PD, Gilas T (1982). "Creation of an intrahepatic portosystemic shunt with a Grüntzig balloon catheter". Can Med Assoc J. 126 (3): 267–8. PMC 1862861. PMID 6977404.
- ↑ Gordon JD, Colapinto RF, Abecassis M, Makowka L, Langer B, Blendis LM, Taylor B, Stronell RD (1987). "Transjugular intrahepatic portosystemic shunt: a nonoperative approach to life-threatening variceal bleeding". Can J Surg. 30 (1): 45–9. PMID 3493058.
- ↑ Palmaz JC, Sibbitt RR, Reuter SR, Garcia F, Tio FO (1985). "Expandable intrahepatic portacaval shunt stents: early experience in the dog". AJR Am J Roentgenol. 145 (4): 821–5. doi:10.2214/ajr.145.4.821. PMID 3876006.
- ↑ Conn HO (1993). "Transjugular intrahepatic portal-systemic shunts: the state of the art". Hepatology. 17 (1): 148–58. PMID 8423036.
- ↑ Nishimine K, Saxon RR, Kichikawa K, Mendel-Hartvig J, Timmermans HA, Shim HJ, Uchida BT, Barton RE, Keller FS, Rösch J (1995). "Improved transjugular intrahepatic portosystemic shunt patency with PTFE-covered stent-grafts: experimental results in swine". Radiology. 196 (2): 341–7. doi:10.1148/radiology.196.2.7617843. PMID 7617843.
- ↑ Guevara M, Rodes J. Hepatorenal syndrome. Int J Biochem Cell Biol. 2005 Jan;37(1):22-6. PMID 15381144.
- ↑ Rössle, Martin (2013). "TIPS: 25years later". Journal of Hepatology. 59 (5): 1081–1093. doi:10.1016/j.jhep.2013.06.014. ISSN 0168-8278.
- ↑ Mallavarapu RK, Grimsley EW (2007). "Incarcerated umbilical hernia after transjugular intrahepatic portosystemic shunt procedure for refractory ascites". Clin. Gastroenterol. Hepatol. 5 (9): A26. doi:10.1016/j.cgh.2007.07.018. PMID 17825762.
- ↑ What You Need to Know about the Transjugular Intrahepatic Portosystemic Shunt (TIPS). Cleveland Clinic. URL: http://www.clevelandclinic.org/health/health-info/docs/0200/0237.asp?index=4956. Accessed: February 19, 2007.