Toxoplasmosis laboratory findings: Difference between revisions
Aditya Ganti (talk | contribs) No edit summary |
Aditya Ganti (talk | contribs) No edit summary |
||
| Line 2: | Line 2: | ||
{{Toxoplasmosis}} | {{Toxoplasmosis}} | ||
{{CMG}} ; {{AE}} {{ADG}} | {{CMG}} ; {{AE}} {{ADG}} | ||
==Overview== | |||
==Laboratory Findings== | ==Laboratory Findings== | ||
===Initial tests=== | |||
Test Result | |||
anti-Toxoplasma IgG (serum) | |||
detectable, with titer | |||
anti-Toxoplasma IgM (serum) | |||
detectable, with titer | |||
CT (with IV contrast) or MR imaging of brain | |||
ring-enhancing brain lesion(s), usually multiple, often involving the basal ganglia | |||
Other Tests to Consider | |||
Test Result | |||
anti-Toxoplasma IgA (serum) | |||
detectable | |||
anti-Toxoplasma IgE (serum) | |||
detectable | |||
Toxoplasma-specific IgG avidity index (serum) | |||
high avidity index indicates a mature IgG response to T gondii and presumes infection is not acute | |||
differential agglutination test (AC/HS) | |||
ratio of AC/HS titer interpreted as acute | |||
PCR (body fluids and tissue) | |||
detection of T gondii DNA in amniotic fluid indicates fetal infection. Detection of T gondii DNA in body fluids in an immunocompromised host establishes infection. Detection of T gondii in vitreous fluid indicates ophthalmic infection | |||
biopsy | |||
cysts, free tachyzoites, inflammatory cells, necrotizing abscesses | |||
===Interpretation of Serological Tests=== | ===Interpretation of Serological Tests=== | ||
{{familytree/start}} | {{familytree/start}} | ||
Revision as of 23:40, 31 May 2017
|
Toxoplasmosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Toxoplasmosis laboratory findings On the Web |
|
American Roentgen Ray Society Images of Toxoplasmosis laboratory findings |
|
Risk calculators and risk factors for Toxoplasmosis laboratory findings |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] ; Associate Editor(s)-in-Chief: Aditya Ganti M.B.B.S. [2]
Overview
Laboratory Findings
Initial tests
Test Result anti-Toxoplasma IgG (serum) detectable, with titer anti-Toxoplasma IgM (serum) detectable, with titer CT (with IV contrast) or MR imaging of brain ring-enhancing brain lesion(s), usually multiple, often involving the basal ganglia Other Tests to Consider Test Result anti-Toxoplasma IgA (serum) detectable anti-Toxoplasma IgE (serum) detectable Toxoplasma-specific IgG avidity index (serum) high avidity index indicates a mature IgG response to T gondii and presumes infection is not acute differential agglutination test (AC/HS) ratio of AC/HS titer interpreted as acute PCR (body fluids and tissue) detection of T gondii DNA in amniotic fluid indicates fetal infection. Detection of T gondii DNA in body fluids in an immunocompromised host establishes infection. Detection of T gondii in vitreous fluid indicates ophthalmic infection biopsy cysts, free tachyzoites, inflammatory cells, necrotizing abscesses
Interpretation of Serological Tests
| IgG/IgM(ideally performed in the first trimester | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Negative IgG and IgM | Positive IgG Negative IgM | Positive IgM Negative IgG | Positive IgG and IgM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ❑ No serologic evidence of Toxoplasma infection ❑ Risk of congenital Toxoplasmosis only if the woman aquires infection during the pregnancy ❑ Counsel about the preventive measures for T.gondii | <18 weeks of gestation Infection aquired in the past and prior to the pregnancy ❑ Risk of infection is zero unless the patient is immunocompromised ≥18 weeks of gestation ❑ It is difficult to establish the timing of infection | Repeat IgG and IgM in 1 to 3weeks | Serum should be sent to reference laboratory for confirmatory testing ❑ If the confirmatory test is positive initiate treatment and if negative follow up for 12 months | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Follow up testing is indicated during gestation to detect seroconversion | ≤ 18 weeks of gestation ❑ No further action indicated >18 weeks of gestation ❑ Compare to previous serological tests and send samples to a reference laboratory to confirm the timing of infection | ❑ Negative IgG and Positive IgM ❑ Does not have clinical relevance[1] | ❑ Positive IgG and IgM ❑ Seroconverted and fetus is at risk ❑ Initiate treatment and consider PCR | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table adopted from Management of Toxoplasma gondii Infection during Pregnancy[2]
Microscopy
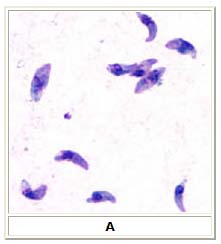
A: Toxoplasma gondii tachyzoites, stained with Giemsa, from a smear of peritoneal fluid obtained from a mouse inoculated with T. gondii. Tachyzoites are typically crescent shaped with a prominent, centrally placed nucleus.
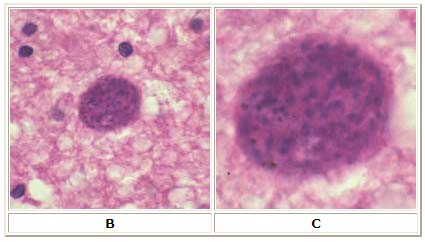
B: Toxoplasma gondii cyst in brain tissue stained with hematoxylin and eosin (100×). C: Zoom of Image B, T. gondii cyst.
References
- ↑ Liesenfeld O, Press C, Montoya JG, Gill R, Isaac-Renton JL, Hedman K; et al. (1997). "False-positive results in immunoglobulin M (IgM) toxoplasma antibody tests and importance of confirmatory testing: the Platelia Toxo IgM test". J Clin Microbiol. 35 (1): 174–8. PMC 229533. PMID 8968902.
- ↑ Montoya, Jose G.; Remington, Jack S. (2008). "Clinical Practice: Management ofToxoplasma gondiiInfection during Pregnancy". Clinical Infectious Diseases. 47 (4): 554–566. doi:10.1086/590149. ISSN 1058-4838.