Toxoplasmosis laboratory findings: Difference between revisions
Aditya Ganti (talk | contribs) No edit summary |
m (Bot: Removing from Primary care) |
||
| (17 intermediate revisions by 3 users not shown) | |||
| Line 2: | Line 2: | ||
{{Toxoplasmosis}} | {{Toxoplasmosis}} | ||
{{CMG}} ; {{AE}} {{ADG}} | {{CMG}} ; {{AE}} {{ADG}} | ||
==Overview== | |||
[[Toxoplasma gondii|Toxoplasma]] infection is diagnosed by the presence of [[Parasites|parasite]] in the fluids such as blood, body fluids, or tissue by [[DNA]] amplification, microscopy or by isolation of the organism. The most commonly used diagnostic test is the [[PCR]] of the amniotic fluid and a positive test is diagnostic of congenital toxoplasmosis. The most commonly used diagnostic investigation for early detection is the [[Serological testing|serological]] detection of antibodies ([[IgG]], [[IgM]] and [[IgA]]) in the serum. A combination of all the [[antibodies]] ([[IgG]], [[IgM]], [[IgA]]) is usally employed.<ref name="pmid10521739">{{cite journal| author=Foulon W, Pinon JM, Stray-Pedersen B, Pollak A, Lappalainen M, Decoster A et al.| title=Prenatal diagnosis of congenital toxoplasmosis: a multicenter evaluation of different diagnostic parameters. | journal=Am J Obstet Gynecol | year= 1999 | volume= 181 | issue= 4 | pages= 843-7 | pmid=10521739 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10521739 }} </ref> | |||
==Laboratory Findings== | ==Laboratory Findings== | ||
[[Toxoplasma gondii|Toxoplasma]] infection is diagnosed by the presence of [[Parasites|parasite]] in the fluids such as blood, body fluids, or tissue by [[DNA]] amplification, microscopy or by isolation of the organism. The most commonly used diagnostic test is the [[PCR]] of the amniotic fluid and a positive test is diagnostic of congenital toxoplasmosis.The most commonly used diagnostic investigation for early detection is the [[Serological testing|serological]] detection of antibodies ([[IgG]], [[IgM]] and [[IgA]]) in the serum. A combination of all the [[antibodies]] ([[IgG]], [[IgM]], [[IgA]]) is usally employed.<ref name="pmid10521739">{{cite journal| author=Foulon W, Pinon JM, Stray-Pedersen B, Pollak A, Lappalainen M, Decoster A et al.| title=Prenatal diagnosis of congenital toxoplasmosis: a multicenter evaluation of different diagnostic parameters. | journal=Am J Obstet Gynecol | year= 1999 | volume= 181 | issue= 4 | pages= 843-7 | pmid=10521739 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10521739 }} </ref> | |||
====Principles and various methods used for the diagnosis of toxoplasmosis:==== | |||
{{ | {| class="wikitable" | ||
!Principle | |||
!Detection | |||
!Method | |||
!Findings supporting the diagnosis of Toxoplasmosis | |||
|- | |||
| rowspan="2" |Toxoplasma specific humoral responses<ref name="pmid26141811">{{cite journal| author=Tanimura K, Nishikawa A, Tairaku S, Shinozaki N, Deguchi M, Morizane M et al.| title=The IgG avidity value for the prediction of Toxoplasma gondii infection in the amniotic fluid. | journal=J Infect Chemother | year= 2015 | volume= 21 | issue= 9 | pages= 668-71 | pmid=26141811 | doi=10.1016/j.jiac.2015.05.013 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26141811 }}</ref> | |||
{ | |[[IgG]], [[IgM]], [[IgA]] | ||
|Dye test, [[ELISA]], ELISA-like assays, immunofluorescence, agglutination | |||
| | |||
*Positive [[IgM]] after 5 days of life and in the absence of blood transfusions | |||
*Positive [[IgA]] after 10 days of life | |||
*Persistence of Toxoplasma [[IgG]] beyond 1 year of age | |||
|- | |||
|[[IgG]], [[IgM]], and [[IgA]] to specific Toxoplasma [[antigen]] | |||
| | |||
[[Western blot]] | |||
| | |||
*Presence of specific bands only seen in the newborn or bands with higher intensity than maternal ones for [[IgG]] and/or [[IgM]] and/or [[IgA]] in a reference laboratory | |||
|- | |||
|Toxoplasma [[nucleic acid amplification]] | |||
|[[DNA]] | |||
|[[PCR]] | |||
| | |||
*Positive result in any body fluid (e.g: [[amniotic fluid]], [[cerebrospinal fluid]], peripheral blood, urine) | |||
|- | |||
|Immunohistochemistry of Toxoplasma specific [[antigens]] in tissue | |||
|Antigens | |||
|Immunoperoxidase | |||
| | |||
*Positive result in any tissue(e.g., [[brain]] or other fetal tissue) | |||
|- | |||
|Visualization by microscopy | |||
|Visual identification of [[tachyzoites]] and/or cysts | |||
|Stains such as hematoxylin/eosin, Giemsa | |||
| | |||
*Positive identification in a reference laboratory | |||
|- | |||
|Isolation of Toxoplasma | |||
|Whole live parasite | |||
|Inoculation in peritoneal cavity of mice | |||
| | |||
*Detection of live cysts from any body fluid or tissue that has been inoculated in mice in a reference laboratory | |||
|} | |||
*A major problem with Toxoplasma-specific [[Immunoglobulin M|IgM]] testing is lack of specificity. | |||
*Two situations occur frequently: i) persons with a positive [[IgM]] but negative [[IgG]], and ii) individuals with positive [[IgG]] and [[IgM]] results. | |||
*In the first situation, a positive [[IgM]] result with a negative [[IgG]] result in the same specimen should be viewed with great suspicion; the patient's blood should be redrawn two weeks after the first and tested together with the first specimen. | |||
*If the first specimen was drawn very early after infection, the patient should have highly positive [[IgG]] and [[IgM]] antibodies in the second sample. | |||
*If the [[IgG]] is negative and the [[IgM]] is positive in both specimens, the [[IgM]] result should be considered to be a false positive and the patient should be considered to be not infected. | |||
*In the second situation, a second specimen should be drawn and both specimens submitted together to a reference lab which employs a different [[IgM]] testing system for confirmation. | |||
* Prior to initiation of patient management for acute toxoplasmosis, all [[IgG]][[IgM|/IgM]] positives should be submitted to a reference lab for [[IgG]] avidity testing. | |||
===Microscopy=== | ===Microscopy=== | ||
'''A''': Toxoplasma gondii tachyzoites, stained with Giemsa, from a smear of peritoneal fluid obtained from a mouse inoculated with T. gondii. Tachyzoites are typically crescent shaped with a prominent, centrally placed nucleus. | '''A''': [[Toxoplasma gondii]] [[Tachyzoites|tachyzoites,]] stained with Giemsa, from a smear of peritoneal fluid obtained from a mouse inoculated with [[Toxoplasma gondii|T. gondii.]] [[Tachyzoites]] are typically crescent shaped with a prominent, centrally placed nucleus. | ||
[[Image:Toxoplasma gondii tachyzoites.jpg|left|Toxoplasma gondii tachyzoites, stained with Giemsa]] | [[Image:Toxoplasma gondii tachyzoites.jpg|left|Toxoplasma gondii tachyzoites, stained with Giemsa]] | ||
<br clear="left"/> | <br clear="left"/> | ||
'''B''': [[Toxoplasma gondii]] cyst in brain tissue stained with hematoxylin and eosin (100×). | |||
'''B''': Toxoplasma gondii cyst in brain tissue stained with hematoxylin and eosin (100×). | |||
'''C''': Zoom of Image B, T. gondii cyst. | '''C''': Zoom of Image B, T. gondii cyst. | ||
[[Image:Toxoplasma gondii cyst.jpg|left|Toxoplasma gondii cyst, hematoxylin and eosin stain]] | [[Image:Toxoplasma gondii cyst.jpg|left|Toxoplasma gondii cyst, hematoxylin and eosin stain]] | ||
<br clear="left"/> | <br clear="left"/> | ||
===Congenital Toxoplasmosis=== | |||
*If the patient is pregnant, and [[IgG]]/[[IgM]] positive, an [[IgG]] avidity test should be performed. | |||
*A high avidity result in the first 12 to 16 weeks of [[pregnancy]] (time dependent upon the commercial test kit) essentially rules out an infection acquired during gestation. | |||
* A low [[IgG]] avidity result should not be interpreted as indicating recent infection because some individuals have persistent low [[IgG]] avidity for many months after infection. | |||
* Suspected recent infection in a pregnant woman should be confirmed prior to intervention by having samples tested at a [[toxoplasmosis]] reference laboratory. | |||
* If the patient has clinical illness compatible with [[toxoplasmosis]] but the [[IgG]] titer is low, a follow-up titer two to three weeks later should show an increase in antibody titer if the illness is due to acute [[toxoplasmosis]], assuming the host is not severely [[immunocompromised]]. | |||
==References== | ==References== | ||
| Line 37: | Line 81: | ||
{{WikiDoc Help Menu}} | {{WikiDoc Help Menu}} | ||
{{WikiDoc Sources}} | {{WikiDoc Sources}} | ||
[[Category:Disease]] | [[Category:Disease]] | ||
[[Category:Gastroenterology]] | [[Category:Gastroenterology]] | ||
[[Category:Neurology]] | [[Category:Neurology]] | ||
[[Category:Neurosurgery]] | [[Category:Neurosurgery]] | ||
| Line 50: | Line 92: | ||
[[Category:Zoonoses]] | [[Category:Zoonoses]] | ||
[[Category:Parasitic diseases]] | [[Category:Parasitic diseases]] | ||
[[Category:Needs content]] | [[Category:Needs content]] | ||
[[Category:Emergency mdicine]] | |||
[[Category:Up-To-Date]] | |||
[[Category:Infectious disease]] | |||
Latest revision as of 00:26, 30 July 2020
|
Toxoplasmosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Toxoplasmosis laboratory findings On the Web |
|
American Roentgen Ray Society Images of Toxoplasmosis laboratory findings |
|
Risk calculators and risk factors for Toxoplasmosis laboratory findings |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] ; Associate Editor(s)-in-Chief: Aditya Ganti M.B.B.S. [2]
Overview
Toxoplasma infection is diagnosed by the presence of parasite in the fluids such as blood, body fluids, or tissue by DNA amplification, microscopy or by isolation of the organism. The most commonly used diagnostic test is the PCR of the amniotic fluid and a positive test is diagnostic of congenital toxoplasmosis. The most commonly used diagnostic investigation for early detection is the serological detection of antibodies (IgG, IgM and IgA) in the serum. A combination of all the antibodies (IgG, IgM, IgA) is usally employed.[1]
Laboratory Findings
Toxoplasma infection is diagnosed by the presence of parasite in the fluids such as blood, body fluids, or tissue by DNA amplification, microscopy or by isolation of the organism. The most commonly used diagnostic test is the PCR of the amniotic fluid and a positive test is diagnostic of congenital toxoplasmosis.The most commonly used diagnostic investigation for early detection is the serological detection of antibodies (IgG, IgM and IgA) in the serum. A combination of all the antibodies (IgG, IgM, IgA) is usally employed.[1]
Principles and various methods used for the diagnosis of toxoplasmosis:
| Principle | Detection | Method | Findings supporting the diagnosis of Toxoplasmosis |
|---|---|---|---|
| Toxoplasma specific humoral responses[2] | IgG, IgM, IgA | Dye test, ELISA, ELISA-like assays, immunofluorescence, agglutination | |
| IgG, IgM, and IgA to specific Toxoplasma antigen | |||
| Toxoplasma nucleic acid amplification | DNA | PCR |
|
| Immunohistochemistry of Toxoplasma specific antigens in tissue | Antigens | Immunoperoxidase |
|
| Visualization by microscopy | Visual identification of tachyzoites and/or cysts | Stains such as hematoxylin/eosin, Giemsa |
|
| Isolation of Toxoplasma | Whole live parasite | Inoculation in peritoneal cavity of mice |
|
- A major problem with Toxoplasma-specific IgM testing is lack of specificity.
- Two situations occur frequently: i) persons with a positive IgM but negative IgG, and ii) individuals with positive IgG and IgM results.
- In the first situation, a positive IgM result with a negative IgG result in the same specimen should be viewed with great suspicion; the patient's blood should be redrawn two weeks after the first and tested together with the first specimen.
- If the first specimen was drawn very early after infection, the patient should have highly positive IgG and IgM antibodies in the second sample.
- If the IgG is negative and the IgM is positive in both specimens, the IgM result should be considered to be a false positive and the patient should be considered to be not infected.
- In the second situation, a second specimen should be drawn and both specimens submitted together to a reference lab which employs a different IgM testing system for confirmation.
- Prior to initiation of patient management for acute toxoplasmosis, all IgG/IgM positives should be submitted to a reference lab for IgG avidity testing.
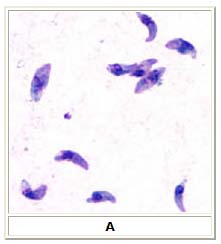
Microscopy
A: Toxoplasma gondii tachyzoites, stained with Giemsa, from a smear of peritoneal fluid obtained from a mouse inoculated with T. gondii. Tachyzoites are typically crescent shaped with a prominent, centrally placed nucleus.
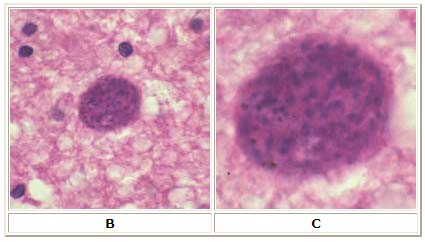
B: Toxoplasma gondii cyst in brain tissue stained with hematoxylin and eosin (100×).
C: Zoom of Image B, T. gondii cyst.
Congenital Toxoplasmosis
- If the patient is pregnant, and IgG/IgM positive, an IgG avidity test should be performed.
- A high avidity result in the first 12 to 16 weeks of pregnancy (time dependent upon the commercial test kit) essentially rules out an infection acquired during gestation.
- A low IgG avidity result should not be interpreted as indicating recent infection because some individuals have persistent low IgG avidity for many months after infection.
- Suspected recent infection in a pregnant woman should be confirmed prior to intervention by having samples tested at a toxoplasmosis reference laboratory.
- If the patient has clinical illness compatible with toxoplasmosis but the IgG titer is low, a follow-up titer two to three weeks later should show an increase in antibody titer if the illness is due to acute toxoplasmosis, assuming the host is not severely immunocompromised.
References
- ↑ 1.0 1.1 Foulon W, Pinon JM, Stray-Pedersen B, Pollak A, Lappalainen M, Decoster A; et al. (1999). "Prenatal diagnosis of congenital toxoplasmosis: a multicenter evaluation of different diagnostic parameters". Am J Obstet Gynecol. 181 (4): 843–7. PMID 10521739.
- ↑ Tanimura K, Nishikawa A, Tairaku S, Shinozaki N, Deguchi M, Morizane M; et al. (2015). "The IgG avidity value for the prediction of Toxoplasma gondii infection in the amniotic fluid". J Infect Chemother. 21 (9): 668–71. doi:10.1016/j.jiac.2015.05.013. PMID 26141811.