Thymoma pathophysiology: Difference between revisions
Amr Marawan (talk | contribs) No edit summary |
Amr Marawan (talk | contribs) No edit summary |
||
| Line 6: | Line 6: | ||
==Overview== | ==Overview== | ||
In 1999, a WHO Working group suggested a non-committal terminology (Masaoka classification), preserving the distinct categories of the histogenetic classification, but using letters and numbers to designate tumour entities. Recently, it has been very well accepted as it provides an easy comparison of clinical, pathological and immunological studies.<ref>{{Cite web | last = | first = | title = http://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb10/BB10.pdf | url = http://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb10/BB10.pdf | publisher = | date = | accessdate = }}</ref> | |||
==Pathophysiology== | ==Pathophysiology== | ||
| Line 11: | Line 12: | ||
==Pathologic Classification== | ==Pathologic Classification== | ||
====Thymic epithelial tumors can be divided according to the degree of differentiation into==== | |||
Thymic epithelial tumors can be divided according to the degree of differentiation into | |||
*Type A. Medullar thymoma: population of neoplastic thymic epithelial cells having a spindle/oval shape, lacking nuclear atypia and accompanied by few or no non-neopl astic lymphocytes. | *Type A. Medullar thymoma: population of neoplastic thymic epithelial cells having a spindle/oval shape, lacking nuclear atypia and accompanied by few or no non-neopl astic lymphocytes. | ||
*Type B. Cortical thymoma: round and polygonal tumour cells.According to the infiltration of lymphocytes and cellular atypia are subclassified into:B1(large lymphocytic infiltrate with epithelial cells, practically indisting uishable from normal thymic cortex tumour that resembles the normal functional thymus), B2 (less lymphocytic infiltrate, the neoplastic epithelial component appears as scattered plump cells with vesicular nuclei and distinct nucleoli, perivascul ar spaces are common and sometimes very prominent with a perivascular arrangement of tumour cells), B3 (epithelial cells having a round or polygonal shape and exhibiting no or mild atypia, admixed with a minor component of lymphocytes, sheet-lik e growth of the neoplastic epithelial cells). | *Type B. Cortical thymoma: round and polygonal tumour cells.According to the infiltration of lymphocytes and cellular atypia are subclassified into:B1(large lymphocytic infiltrate with epithelial cells, practically indisting uishable from normal thymic cortex tumour that resembles the normal functional thymus), B2 (less lymphocytic infiltrate, the neoplastic epithelial component appears as scattered plump cells with vesicular nuclei and distinct nucleoli, perivascul ar spaces are common and sometimes very prominent with a perivascular arrangement of tumour cells), B3 (epithelial cells having a round or polygonal shape and exhibiting no or mild atypia, admixed with a minor component of lymphocytes, sheet-lik e growth of the neoplastic epithelial cells). | ||
| Line 19: | Line 19: | ||
According to different published series, it is estimated that sub-types B2 and AB are the most common (20–35%) while A and B1 subtypes have a much lower incidence (5–10%).<ref name="www.ncbi.nlm.nih.gov">{{Cite web | last = | first = | title = WHO histologic classification is a prognosti... [Ann Thorac Surg. 2004] - PubMed - NCBI | url = http://www.ncbi.nlm.nih.gov/pubmed/15063231 | publisher = | date = | accessdate = }}</ref> | According to different published series, it is estimated that sub-types B2 and AB are the most common (20–35%) while A and B1 subtypes have a much lower incidence (5–10%).<ref name="www.ncbi.nlm.nih.gov">{{Cite web | last = | first = | title = WHO histologic classification is a prognosti... [Ann Thorac Surg. 2004] - PubMed - NCBI | url = http://www.ncbi.nlm.nih.gov/pubmed/15063231 | publisher = | date = | accessdate = }}</ref> | ||
====Masaoka-Koga Staging System==== | |||
{| | |||
| [[File:masaoka.png|800px|thumb]] | |||
|}<ref>{{Cite web | last = | first = | title = https://ccehub.org/resources/376/download/the_masaoka_koga_system_12-21-10_1.pdf | url = https://ccehub.org/resources/376/download/the_masaoka_koga_system_12-21-10_1.pdf | publisher = | date = | accessdate = }}</ref> | |||
<gallery> | <gallery> | ||
Image:T003.png| Histopathological image of [[Thymoma]] type B1. [[Anterior mediastinal mass]] surgically resected. Hematoxylin & eosin stain | Image:T003.png| Histopathological image of [[Thymoma]] type B1. [[Anterior mediastinal mass]] surgically resected. Hematoxylin & eosin stain | ||
Revision as of 21:03, 21 February 2014
|
Thymoma Microchapters |
|
Diagnosis |
|---|
|
Case Studies |
|
Thymoma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Thymoma pathophysiology |
|
Risk calculators and risk factors for Thymoma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Amr Marawan, M.D. [2]
Please help WikiDoc by adding more content here. It's easy! Click here to learn about editing.
Overview
In 1999, a WHO Working group suggested a non-committal terminology (Masaoka classification), preserving the distinct categories of the histogenetic classification, but using letters and numbers to designate tumour entities. Recently, it has been very well accepted as it provides an easy comparison of clinical, pathological and immunological studies.[1]
Pathophysiology
Thymoma originates from the epithelial cell population in the thymus. Many subtypes are recognized, some of which have a better- or worse-than-general prognosis.[2]
Pathologic Classification
Thymic epithelial tumors can be divided according to the degree of differentiation into
- Type A. Medullar thymoma: population of neoplastic thymic epithelial cells having a spindle/oval shape, lacking nuclear atypia and accompanied by few or no non-neopl astic lymphocytes.
- Type B. Cortical thymoma: round and polygonal tumour cells.According to the infiltration of lymphocytes and cellular atypia are subclassified into:B1(large lymphocytic infiltrate with epithelial cells, practically indisting uishable from normal thymic cortex tumour that resembles the normal functional thymus), B2 (less lymphocytic infiltrate, the neoplastic epithelial component appears as scattered plump cells with vesicular nuclei and distinct nucleoli, perivascul ar spaces are common and sometimes very prominent with a perivascular arrangement of tumour cells), B3 (epithelial cells having a round or polygonal shape and exhibiting no or mild atypia, admixed with a minor component of lymphocytes, sheet-lik e growth of the neoplastic epithelial cells).
- Type AB. Combined areas of A and B characteristics (foci having the features of type A thymoma are admixed with foci rich in lymphocytes).
- Type C. Thymic carcinoma: clear-cut cytologic atypia and a set of cytoarchitectural features no longer specific to the thymus but rather analogous to those seen in carcinomas of other organs, lack immature lymphocytes.
According to different published series, it is estimated that sub-types B2 and AB are the most common (20–35%) while A and B1 subtypes have a much lower incidence (5–10%).[3]
Masaoka-Koga Staging System
 |
-
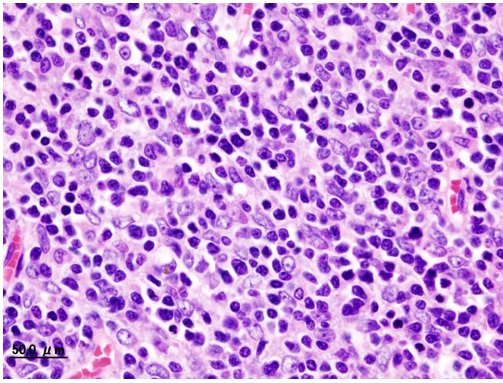
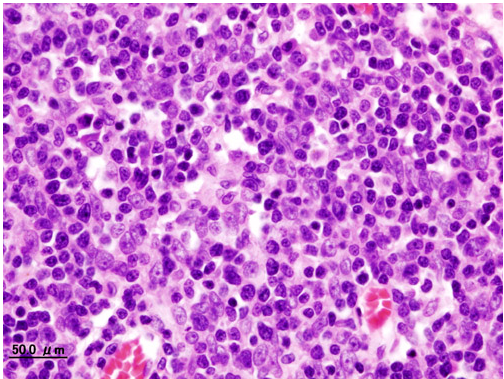
Histopathological image of Thymoma type B1. Anterior mediastinal mass surgically resected. Hematoxylin & eosin stain
-
Histopathological image representing a noninvasive Thymoma type B1, surgically resected. Hematoxylin & eosin
-
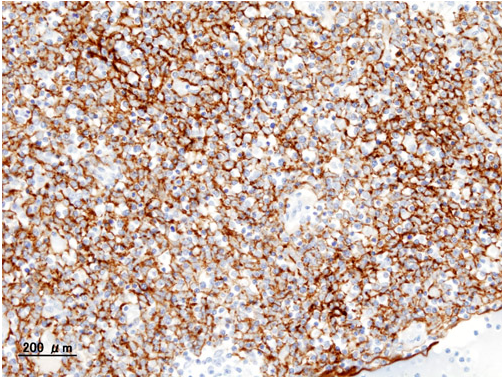
Histopathological image of Thymoma type B1. Anterior mediastinal mass surgically resected. Cytokeratin CAM5.2 immunostain
-
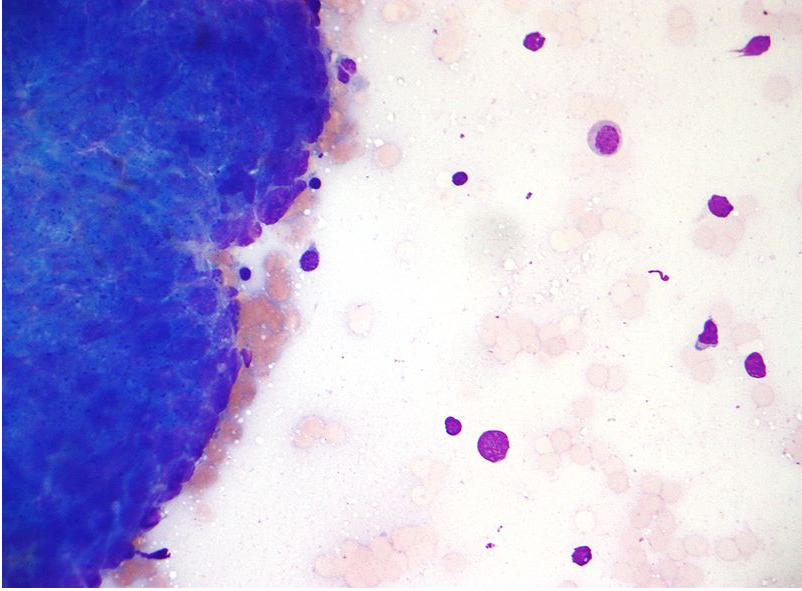
Micrograph of a Thymoma. FNA specimen. Field stain
-
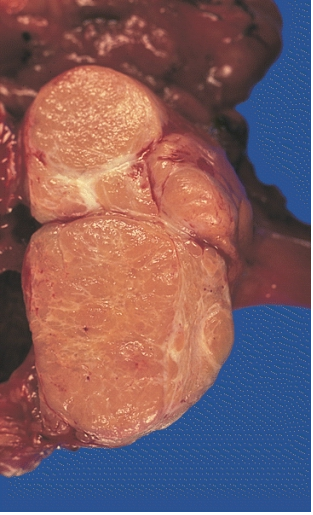
MEDIASTINUM: LOCALLY INVASIVE, CIRCUMSCRIBED THYMOMA (MIXED LYMPHOCYTIC AND EPITHELIAL AND MIXED POLYGONAL AND SPINDLE).Its cut surface bulges, and is pale tan and faintly lobulated. It invaded the capsule at a few points but still remained within the thymus
Associated Disorders
A third of the patients who have a thymoma detected because they have an associated autoimmune disorder. The most common condition in this group is myasthenia gravis (of which 25-50% are associated with a thymoma); patients with myasthenia are routinely screened for thymoma. Other associated autoimmune conditions are pure red cell aplasia and Good's syndrome (thymoma with combined immunodeficiency and hypoimmunoglobulinemia G). Rare associations that have been reported are: acute pericarditis, Addison's disease, agranulocytosis, alopecia areata, ulcerative colitis, Cushing's disease, hemolytic anemia, limbic encephalopathy, myocarditis, nephrotic syndrome, panhypopituitarism, pernicious anemia, polymyositis, rheumatoid arthritis, sarcoidosis, scleroderma, sensorimotor radiculopathy, stiff person syndrome, systemic lupus erythematosus and thyroiditis.[2]
References
- ↑ "http://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb10/BB10.pdf" (PDF). External link in
|title=(help) - ↑ 2.0 2.1 Thomas CR, Wright CD, Loehrer PJ (1999). "Thymoma: state of the art". Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 17 (7): 2280–9. PMID 10561285. Retrieved 2012-01-18. Unknown parameter
|month=ignored (help) - ↑ "WHO histologic classification is a prognosti... [Ann Thorac Surg. 2004] - PubMed - NCBI".
- ↑ "https://ccehub.org/resources/376/download/the_masaoka_koga_system_12-21-10_1.pdf" (PDF). External link in
|title=(help)