Thrombin human: Difference between revisions
Gloria Picoy (talk | contribs) (Created page with "{{DrugProjectFormSinglePage |authorTag={{GP}} |genericName=Thrombin human |aOrAn=an |drugClass=hemostatic |indicationType=treatment |indication=hemorrhage |adverseReactions=pr...") |
Gloria Picoy (talk | contribs) No edit summary |
||
| Line 29: | Line 29: | ||
* The risk of transmitting an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections and by inactivating and removing certain viruses. Despite these measures, such products can still potentially transmit disease. | * The risk of transmitting an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections and by inactivating and removing certain viruses. Despite these measures, such products can still potentially transmit disease. | ||
* There is also the possibility that unknown infectious agents may be present in such products. | * There is also the possibility that unknown infectious agents may be present in such products. | ||
|clinicalTrials=* The most common adverse reactions during clinical trial (reported in at least 2% of subjects treated with | |clinicalTrials=* The most common adverse reactions during clinical trial (reported in at least 2% of subjects treated with thrombin human) were prolonged activated partial thromboplastin time, increased INR, decreased lymphocyte count, prolonged prothrombin time and increased neutrophil count. | ||
| Line 36: | Line 36: | ||
* Anaphylactic reactions may occur in rare cases. No adverse events of this type were reported during the conduct of the clinical trials. Mild reactions can be managed with anti-histamines. Severe hypotensive reactions require immediate intervention using current principles of shock therapy. | * Anaphylactic reactions may occur in rare cases. No adverse events of this type were reported during the conduct of the clinical trials. Mild reactions can be managed with anti-histamines. Severe hypotensive reactions require immediate intervention using current principles of shock therapy. | ||
* In a phase III multicenter, prospective, controlled, randomized, double-blinded study of 305 subjects where | * In a phase III multicenter, prospective, controlled, randomized, double-blinded study of 305 subjects where thrombin human (n=153) was compared with bovine thrombin (n=152), occurrence of adverse events was not statistically different between the two groups. | ||
* Overall, adverse events occurred in similar proportions of subjects in the two study groups. No clinically significant differences were seen in age (<65 years, >65 years) or gender subgroup analyses of adverse events. | * Overall, adverse events occurred in similar proportions of subjects in the two study groups. No clinically significant differences were seen in age (<65 years, >65 years) or gender subgroup analyses of adverse events. | ||
* At least one serious adverse event (SAE) was reported for 26/153 (17%) subjects treated with human thrombin and 17/152 (11%) subjects treated with bovine thrombin. The SAEs reported were associated with post-surgical complications (e.g. wound infection 3/153 for | * At least one serious adverse event (SAE) was reported for 26/153 (17%) subjects treated with human thrombin and 17/152 (11%) subjects treated with bovine thrombin. The SAEs reported were associated with post-surgical complications (e.g. wound infection 3/153 for thrombin human and 2/152 for bovine thrombin) and the medical condition of the subject and were not considered related to study drug. Two subjects (1.3%) in thrombin human group experienced a treatment emergent severe adverse event: respiratory arrest and post-procedural hematoma (in one subject) and extradural hematoma. Three subjects in the bovine thrombin group experienced a treatment emergent severe adverse event: hyperhidrosis, pyrexia and post-procedural hematoma. | ||
No deaths were reported during the study period. | No deaths were reported during the study period. | ||
Viral serology was not monitored during the study with | Viral serology was not monitored during the study with thrombin human. However, no adverse events indicative of infection with transfusion-transmissible agents were reported. | ||
[[File:Thrombin human adverse reactions.png|thumb|none|500px]] | [[File:Thrombin human adverse reactions.png|thumb|none|500px]] | ||
| Line 51: | Line 51: | ||
In the clinical study, serum samples were collected at baseline and at 5 weeks post-surgery for evaluation of antibodies to bovine thrombin, bovine Factor V/Va, human thrombin, and human Factor V/Va. Samples were collected at both time points for 81.3% of the subjects. The ELISA data were adjudicated by a panel of experts blinded to treatment assignment. After reviewing all data, the panel used an algorithm for assigning outcomes for each antigen: seroconversion negative or seroconversion positive. | In the clinical study, serum samples were collected at baseline and at 5 weeks post-surgery for evaluation of antibodies to bovine thrombin, bovine Factor V/Va, human thrombin, and human Factor V/Va. Samples were collected at both time points for 81.3% of the subjects. The ELISA data were adjudicated by a panel of experts blinded to treatment assignment. After reviewing all data, the panel used an algorithm for assigning outcomes for each antigen: seroconversion negative or seroconversion positive. | ||
The protocol did not specify any comparative analysis for immunogenicity data, only descriptive statistics. The adjudicated results show that 3.3% of the subjects treated with | The protocol did not specify any comparative analysis for immunogenicity data, only descriptive statistics. The adjudicated results show that 3.3% of the subjects treated with thrombin human (frozen formulation) developed antibodies to any of the four antigens, compared to 12.7% of the subjects developing antibodies in the control group (bovine thrombin). 7.94% of the subjects treated with bovine thrombin (control group) developed antibodies to bovine thrombin and 9.52% of these subjects developed antibodies to bovine Factor V/Va. A few control subjects had antibodies that cross-reacted with human thrombin, but none had antibodies that cross-reacted with human Factor V/Va. None of the patients treated with thrombin human developed detectable antibodies to human thrombin or to human Factor V/Va. | ||
The detection of antibody formation is highly dependent upon the sensitivity and specificity of the assay. The observed incidence of a positive signal in an assay may be influenced by several factors including timing of sampling, sample handling, concomitant medications, or underlying disease. Therefore, direct comparison of incidence of antibody development to human thrombin, bovine thrombin, human Factor V/Va or bovine Factor V/Va following administration of | The detection of antibody formation is highly dependent upon the sensitivity and specificity of the assay. The observed incidence of a positive signal in an assay may be influenced by several factors including timing of sampling, sample handling, concomitant medications, or underlying disease. Therefore, direct comparison of incidence of antibody development to human thrombin, bovine thrombin, human Factor V/Va or bovine Factor V/Va following administration of thrombin human with incidence of antibody development following administration of other products may be misleading and the clinical significance of these findings is unknown. | ||
|postmarketing=No adverse reactions have been identified from spontaneous post-marketing reports. | |postmarketing=No adverse reactions have been identified from spontaneous post-marketing reports. | ||
|drugInteractions=No drug interactions are known. | |drugInteractions=No drug interactions are known. | ||
|alcohol=Alcohol-Thrombin human interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Thrombin human interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 15:26, 12 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Thrombin human is an hemostatic that is FDA approved for the treatment of hemorrhage. Common adverse reactions include prolonged activated partial thromboplastin time, increased INR, decreased lymphocyte count, prolonged prothrombin time and increased neutrophil count.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Is indicated as an aid to hemostasis whenever oozing blood and minor bleeding from capillaries and small venules.
- Apply only on the surface of bleeding tissue
- The amount of thrombin human required depends upon the area of tissue to be treated and the method of application.
- As an approximate guide, volumes up to 10 ml were used in clinical studies where thrombin human was used in conjunction with absorbable gelatin sponge.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Thrombin human in adult patients.
Non–Guideline-Supported Use
- Peptic ulcer with hemorrhage
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Apply only on the surface of bleeding tissue
- The amount of thrombin human required depends upon the area of tissue to be treated and the method of application.
- As an approximate guide, volumes up to 10 ml were used in clinical studies where thrombin human was used in conjunction with absorbable gelatin sponge.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Thrombin human in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Thrombin human in pediatric patients.
Contraindications
- Do not use in individuals known to have anaphylactic or severe systemic reaction to human blood products.
- Do not use for the treatment of severe or brisk arterial bleeding.
Warnings
Thrombosis
- Potential risk of thrombosis if absorbed systemically
Transmission of Infectious Agents
- Because this product is made from human plasma, it may carry a risk of transmitting infectious agents, such as viruses, and theoretically, the Creutzfeldt-Jakob disease (CJD) agent.
- The risk of transmitting an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections and by inactivating and removing certain viruses. Despite these measures, such products can still potentially transmit disease.
- There is also the possibility that unknown infectious agents may be present in such products.
Adverse Reactions
Clinical Trials Experience
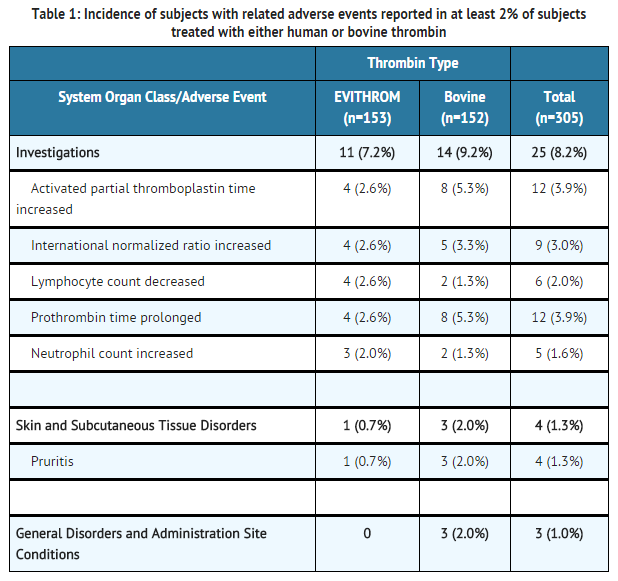
- The most common adverse reactions during clinical trial (reported in at least 2% of subjects treated with thrombin human) were prolonged activated partial thromboplastin time, increased INR, decreased lymphocyte count, prolonged prothrombin time and increased neutrophil count.
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug product cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- Anaphylactic reactions may occur in rare cases. No adverse events of this type were reported during the conduct of the clinical trials. Mild reactions can be managed with anti-histamines. Severe hypotensive reactions require immediate intervention using current principles of shock therapy.
- In a phase III multicenter, prospective, controlled, randomized, double-blinded study of 305 subjects where thrombin human (n=153) was compared with bovine thrombin (n=152), occurrence of adverse events was not statistically different between the two groups.
- Overall, adverse events occurred in similar proportions of subjects in the two study groups. No clinically significant differences were seen in age (<65 years, >65 years) or gender subgroup analyses of adverse events.
- At least one serious adverse event (SAE) was reported for 26/153 (17%) subjects treated with human thrombin and 17/152 (11%) subjects treated with bovine thrombin. The SAEs reported were associated with post-surgical complications (e.g. wound infection 3/153 for thrombin human and 2/152 for bovine thrombin) and the medical condition of the subject and were not considered related to study drug. Two subjects (1.3%) in thrombin human group experienced a treatment emergent severe adverse event: respiratory arrest and post-procedural hematoma (in one subject) and extradural hematoma. Three subjects in the bovine thrombin group experienced a treatment emergent severe adverse event: hyperhidrosis, pyrexia and post-procedural hematoma.
No deaths were reported during the study period.
Viral serology was not monitored during the study with thrombin human. However, no adverse events indicative of infection with transfusion-transmissible agents were reported.
Immunogenicity
In the clinical study, serum samples were collected at baseline and at 5 weeks post-surgery for evaluation of antibodies to bovine thrombin, bovine Factor V/Va, human thrombin, and human Factor V/Va. Samples were collected at both time points for 81.3% of the subjects. The ELISA data were adjudicated by a panel of experts blinded to treatment assignment. After reviewing all data, the panel used an algorithm for assigning outcomes for each antigen: seroconversion negative or seroconversion positive.
The protocol did not specify any comparative analysis for immunogenicity data, only descriptive statistics. The adjudicated results show that 3.3% of the subjects treated with thrombin human (frozen formulation) developed antibodies to any of the four antigens, compared to 12.7% of the subjects developing antibodies in the control group (bovine thrombin). 7.94% of the subjects treated with bovine thrombin (control group) developed antibodies to bovine thrombin and 9.52% of these subjects developed antibodies to bovine Factor V/Va. A few control subjects had antibodies that cross-reacted with human thrombin, but none had antibodies that cross-reacted with human Factor V/Va. None of the patients treated with thrombin human developed detectable antibodies to human thrombin or to human Factor V/Va.
The detection of antibody formation is highly dependent upon the sensitivity and specificity of the assay. The observed incidence of a positive signal in an assay may be influenced by several factors including timing of sampling, sample handling, concomitant medications, or underlying disease. Therefore, direct comparison of incidence of antibody development to human thrombin, bovine thrombin, human Factor V/Va or bovine Factor V/Va following administration of thrombin human with incidence of antibody development following administration of other products may be misleading and the clinical significance of these findings is unknown.
Postmarketing Experience
No adverse reactions have been identified from spontaneous post-marketing reports.
Drug Interactions
No drug interactions are known.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Thrombin human in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Thrombin human in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Thrombin human during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Thrombin human in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Thrombin human in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Thrombin human in geriatric settings.
Gender
There is no FDA guidance on the use of Thrombin human with respect to specific gender populations.
Race
There is no FDA guidance on the use of Thrombin human with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Thrombin human in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Thrombin human in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Thrombin human in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Thrombin human in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Thrombin human Administration in the drug label.
Monitoring
There is limited information regarding Thrombin human Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Thrombin human and IV administrations.
Overdosage
There is limited information regarding Thrombin human overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Thrombin human Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Thrombin human Mechanism of Action in the drug label.
Structure
There is limited information regarding Thrombin human Structure in the drug label.
Pharmacodynamics
There is limited information regarding Thrombin human Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Thrombin human Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Thrombin human Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Thrombin human Clinical Studies in the drug label.
How Supplied
There is limited information regarding Thrombin human How Supplied in the drug label.
Storage
There is limited information regarding Thrombin human Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Thrombin human |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Thrombin human |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Thrombin human Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Thrombin human interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Thrombin human Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Thrombin human Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.