Takayasu's arteritis pathophysiology: Difference between revisions
No edit summary |
No edit summary |
||
| (37 intermediate revisions by 4 users not shown) | |||
| Line 2: | Line 2: | ||
{{Takayasu's arteritis}} | {{Takayasu's arteritis}} | ||
{{CMG}} {{AE}} {{FKH}} | {{CMG}} {{AE}} {{FKH}} | ||
==Overview== | ==Overview== | ||
The [[pathogenesis]] of Takayasu's arteritis is poorly understood. Takayasu's arteritis characterized by segmental and patchy [[Granuloma|granulomatous]] [[inflammation]] of the [[aorta]] and its major derivative branches.This [[inflammation]] leads to [[Artery|arterial]] [[stenosis]], [[thrombosis]], and [[Aneurysm|aneurysms]]. Three factors that have been suggested to have association with susceptibility, development and progression of Takayasu's arteritis are [[Genetics|genetic]] influences, [[Immunology|immunologic]] mechanisms, and relationship to [[tuberculosis]]. The most important conditions associated with Takayasu's arteritis include [[ankylosing spondylitis]], [[inflammatory bowel disease]], and [[Behçet's disease|Behçet's syndrome]]. On gross pathology, stiff and rigid [[aorta]] on [[palpation]], gelatinous appearance of thickened [[adventitia]], and sharp line of demarcation between normal and diseased segments might be seen. On microscopic [[histopathological]] analysis characteristic findings of Takayasu's arteritis include [[inflammation]] around the [[vasa vasorum]] and at the medio-adventitial junction, [[edema]] of the [[Tunica media|media]] and [[adventitia]], [[Large cell|giant cell]] [[Granuloma|granulomatous]] reaction, [[laminar necrosis]], and [[Fragmentation (reproduction)|fragmentation]] of [[Elastic fiber|elastic fibers]]. | |||
==Pathophysiology== | ==Pathophysiology== | ||
* The pathogenesis of Takayasu's arteritis is poorly understood.<ref name="pmid10737351">{{cite journal |vauthors=Inder SJ, Bobryshev YV, Cherian SM, Wang AY, Lord RS, Masuda K, Yutani C |title=Immunophenotypic analysis of the aortic wall in Takayasu's arteritis: involvement of lymphocytes, dendritic cells and granulocytes in immuno-inflammatory reactions |journal=Cardiovasc Surg |volume=8 |issue=2 |pages=141–8 |date=March 2000 |pmid=10737351 |doi= |url=}}</ref> | * The [[pathogenesis]] of Takayasu's arteritis is poorly understood.<ref name="pmid10737351">{{cite journal |vauthors=Inder SJ, Bobryshev YV, Cherian SM, Wang AY, Lord RS, Masuda K, Yutani C |title=Immunophenotypic analysis of the aortic wall in Takayasu's arteritis: involvement of lymphocytes, dendritic cells and granulocytes in immuno-inflammatory reactions |journal=Cardiovasc Surg |volume=8 |issue=2 |pages=141–8 |date=March 2000 |pmid=10737351 |doi= |url=}}</ref> | ||
* | * Granulomatous [[inflammation]] of the [[aorta]] and its major branches might lead to Takayasu's arteritis. | ||
* [[Cell-mediated immunity|Cell-mediated]] mechanisms are considered as a main pathogenesis mechanism of Takayasu's arteritis and it is similar to [[giant cell arteritis]]. | |||
* This [[inflammation]] leads to [[arterial]] [[stenosis]], [[thrombosis]], and [[Aneurysm|aneurysms]]. | |||
* Irregular [[fibrosis]] of the [[Blood vessel|blood vessels]] due to chronic [[vasculitis]] may lead to [[Tunica intima|intimal]] [[fibrosis]]. | |||
* There are three factors that have associated with disease susceptibility, development and progression: | |||
** Relationship to [[tuberculosis]] (TB) | ** Relationship to [[tuberculosis]] (TB) | ||
** Genetic influences | ** [[Genetics|Genetic]] influences | ||
** Immunologic mechanisms | ** [[Immunology|Immunologic]] mechanisms | ||
'''Relationship to tuberculosis (TB)''' | '''Relationship to tuberculosis (TB)''' | ||
* It has been suggested that Takayasu arteritis is associated with TB. Following evidences support this [[hypothesis]]:<ref name="pmid12655">{{cite journal |vauthors=Lupi-Herrera E, Sánchez-Torres G, Marcushamer J, Mispireta J, Horwitz S, Vela JE |title=Takayasu's arteritis. Clinical study of 107 cases |journal=Am. Heart J. |volume=93 |issue=1 |pages=94–103 |date=January 1977 |pmid=12655 |doi= |url=}}</ref> | |||
[[Granulomatous]] inflammation with the Langhans-type of | ** [[Granulomatous]] [[Inflammation|inflammation]] with the [[Langhans giant cells|Langhans-type of giant cells]] in many cases of Takayasu arteritis | ||
** Intermittent coexistence of Takayasu arteritis with [[Lung|pulmonary]] and [[extrapulmonary tuberculosis]] | |||
** [[Hypersensitivity]] to the [[tuberculosis]] organism | |||
'''Genetic influences''' | '''Genetic influences''' | ||
* Geographic distribution of Takayasu arteritis, with high [[prevalence]] in Japan and Korea, suggests that [[Genetics|genetic]] factors are probably play a role in the [[pathogenesis]] of Takayasu arteritis. | |||
* Takayasu arteritis has been associated with different [[human leukocyte antigen]] ([[Human leukocyte antigen|HLA]]) [[Allele|alleles]] in different populations. In Japan and Korea, there is a clear association with the extended [[haplotype]]:<ref name="pmid10980348">{{cite journal |vauthors=Salazar M, Varela A, Ramirez LA, Uribe O, Vasquez G, Egea E, Yunis EJ, Iglesias-Gamarra A |title=Association of HLA-DRB1*1602 and DRB1*1001 with Takayasu arteritis in Colombian mestizos as markers of Amerindian ancestry |journal=Int. J. Cardiol. |volume=75 Suppl 1 |issue= |pages=S113–6 |date=August 2000 |pmid=10980348 |doi= |url=}}</ref> | |||
**[[HLA-B]]*52 | |||
**[[HLA-DRB1]]*1502 | |||
**[[HLA-DRB5]]*0102 | |||
**[[HLA-DQA1]]*0103 | |||
**[[HLA-DQB1]]*0601 | |||
**[[HLA-DP]]A1*02 | |||
**[[HLA-DPB1]]*0901 | |||
'''Immunologic mechanisms''' | '''Immunologic mechanisms''' | ||
* | Because of rheumatic-type complaints in many Takayasu arteritis patients, the relationship between Takayasu arteritis and [[Autoimmunity|autoimmune]] and [[collagen]] [[vascular]] disorders has been suggested. | ||
* | * Immunohistopathologic examination has shown that the infiltrating cells in [[Aorta|aortic]] tissue mainly consist of [[Natural killer cell|killer cells]], especially gamma delta [[T lymphocytes]]. | ||
* These cells may cause [[vascular injury]] by releasing large amounts of the cytolytic compound [[perforin]]. | |||
* It has been reported that γδT cells, αβT cells ([[CD4]] and [[CD8]]), and [[Natural killer cell|natural killer cells]] play an important role in the [[vascular injury]].<ref name="pmid10980341">{{cite journal |vauthors=Seko Y, Takahashi N, Tada Y, Yagita H, Okumura K, Nagai R |title=Restricted usage of T-cell receptor Vgamma-Vdelta genes and expression of costimulatory molecules in Takayasu's arteritis |journal=Int. J. Cardiol. |volume=75 Suppl 1 |issue= |pages=S77–83; discussion S85–7 |date=August 2000 |pmid=10980341 |doi= |url=}}</ref> | |||
* No specific [[Autoantigen|autoantigens]] have yet been identified. | |||
== Associations == | |||
* The most important conditions associated with Takayasu's arteritis include: | * The most important conditions associated with Takayasu's arteritis include: | ||
** Ankylosing spondylitis | ** [[Ankylosing spondylitis]] | ||
** Inflammatory bowel | ** [[Inflammatory bowel disease]] (IBD) | ||
** Behçet's syndrome | ** [[Behçet's disease|Behçet's syndrome]] | ||
== Gross pathology == | |||
On gross pathology characteristic findings of Takayasu's arteritis are as follows:<ref name="pmid10980333">{{cite journal |vauthors=Gravanis MB |title=Giant cell arteritis and Takayasu aortitis: morphologic, pathogenetic and etiologic factors |journal=Int. J. Cardiol. |volume=75 Suppl 1 |issue= |pages=S21–33; discussion S35–6 |date=August 2000 |pmid=10980333 |doi= |url=}}</ref> | |||
* Stiff and rigid [[aorta]] on [[palpation]] | |||
* Gelatinous appearance of thickened [[adventitia]] | |||
* Enlarged [[Paraaortic lymph node|para-aortic]] [[Lymph node|lymph nodes]] in the area of [[Renal artery|renal]] and [[Subclavian artery|subclavian arteries]] | |||
* [[Glycosaminoglycan|<nowiki/><nowiki/>]]Sharp line of demarcation between normal and diseased segments[[Glycosaminoglycan|<nowiki/><nowiki/>]] | |||
== Microscopic pathology == | |||
On microscopic [[histopathological]] analysis characteristic findings of Takayasu's arteritis are as follows:<ref name="pmid10980333" /> | |||
* [[Inflammation]] around the [[vasa vasorum]] and at the medio-adventitial junction | |||
* [[Edema]] of the [[Tunica media|media]] and [[adventitia]] | |||
* [[Large cell|Giant cell]] [[Granuloma|granulomatous]] reaction | |||
* [[Laminar necrosis]] | |||
* [[Fragmentation (biology)|Fragmentation]] of [[Elastic fiber|elastic fibers]] | |||
* Rapid or more severe [[inflammation]] leads to: | |||
** Loss of [[smooth muscle cell]]<nowiki/>s | |||
** Medial weakening | |||
** [[Vascular]] dilatation | |||
** [[Aneurysm]] formation | |||
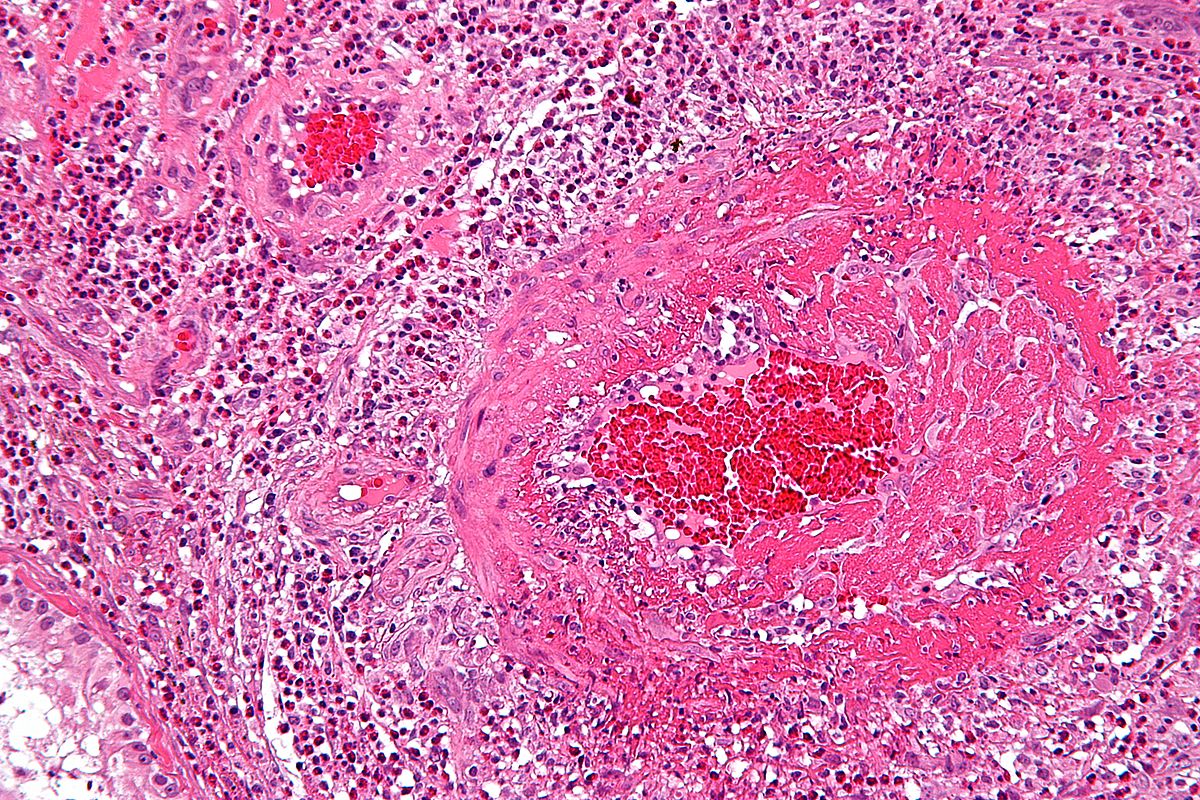
[[Image:Vasculitis.jpg|300px|left|thumb|H & E microscopy of a vessel showing vasculitis (inflammatory cells within the vessel wall), source:https://librepathology.org/wiki/Vasculitides#Takayasu_arteritis]] | |||
<br style="clear:left"> | |||
==References== | ==References== | ||
{{reflist|2}} | {{reflist|2}} | ||
[[Category: | [[Category:Medicine]] | ||
[[Category:Rheumatology]] | [[Category:Rheumatology]] | ||
[[Category: | [[Category:Up-To-Date]] | ||
{{WH}} | {{WH}} | ||
{{WS}} | {{WS}} | ||
Latest revision as of 13:19, 24 May 2018
|
Takayasu's arteritis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Takayasu's arteritis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Takayasu's arteritis pathophysiology |
|
Risk calculators and risk factors for Takayasu's arteritis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Farnaz Khalighinejad, MD [2]
Overview
The pathogenesis of Takayasu's arteritis is poorly understood. Takayasu's arteritis characterized by segmental and patchy granulomatous inflammation of the aorta and its major derivative branches.This inflammation leads to arterial stenosis, thrombosis, and aneurysms. Three factors that have been suggested to have association with susceptibility, development and progression of Takayasu's arteritis are genetic influences, immunologic mechanisms, and relationship to tuberculosis. The most important conditions associated with Takayasu's arteritis include ankylosing spondylitis, inflammatory bowel disease, and Behçet's syndrome. On gross pathology, stiff and rigid aorta on palpation, gelatinous appearance of thickened adventitia, and sharp line of demarcation between normal and diseased segments might be seen. On microscopic histopathological analysis characteristic findings of Takayasu's arteritis include inflammation around the vasa vasorum and at the medio-adventitial junction, edema of the media and adventitia, giant cell granulomatous reaction, laminar necrosis, and fragmentation of elastic fibers.
Pathophysiology
- The pathogenesis of Takayasu's arteritis is poorly understood.[1]
- Granulomatous inflammation of the aorta and its major branches might lead to Takayasu's arteritis.
- Cell-mediated mechanisms are considered as a main pathogenesis mechanism of Takayasu's arteritis and it is similar to giant cell arteritis.
- This inflammation leads to arterial stenosis, thrombosis, and aneurysms.
- Irregular fibrosis of the blood vessels due to chronic vasculitis may lead to intimal fibrosis.
- There are three factors that have associated with disease susceptibility, development and progression:
- Relationship to tuberculosis (TB)
- Genetic influences
- Immunologic mechanisms
Relationship to tuberculosis (TB)
- It has been suggested that Takayasu arteritis is associated with TB. Following evidences support this hypothesis:[2]
- Granulomatous inflammation with the Langhans-type of giant cells in many cases of Takayasu arteritis
- Intermittent coexistence of Takayasu arteritis with pulmonary and extrapulmonary tuberculosis
- Hypersensitivity to the tuberculosis organism
Genetic influences
- Geographic distribution of Takayasu arteritis, with high prevalence in Japan and Korea, suggests that genetic factors are probably play a role in the pathogenesis of Takayasu arteritis.
- Takayasu arteritis has been associated with different human leukocyte antigen (HLA) alleles in different populations. In Japan and Korea, there is a clear association with the extended haplotype:[3]
Immunologic mechanisms
Because of rheumatic-type complaints in many Takayasu arteritis patients, the relationship between Takayasu arteritis and autoimmune and collagen vascular disorders has been suggested.
- Immunohistopathologic examination has shown that the infiltrating cells in aortic tissue mainly consist of killer cells, especially gamma delta T lymphocytes.
- These cells may cause vascular injury by releasing large amounts of the cytolytic compound perforin.
- It has been reported that γδT cells, αβT cells (CD4 and CD8), and natural killer cells play an important role in the vascular injury.[4]
- No specific autoantigens have yet been identified.
Associations
- The most important conditions associated with Takayasu's arteritis include:
Gross pathology
On gross pathology characteristic findings of Takayasu's arteritis are as follows:[5]
- Stiff and rigid aorta on palpation
- Gelatinous appearance of thickened adventitia
- Enlarged para-aortic lymph nodes in the area of renal and subclavian arteries
- Sharp line of demarcation between normal and diseased segments
Microscopic pathology
On microscopic histopathological analysis characteristic findings of Takayasu's arteritis are as follows:[5]
- Inflammation around the vasa vasorum and at the medio-adventitial junction
- Edema of the media and adventitia
- Giant cell granulomatous reaction
- Laminar necrosis
- Fragmentation of elastic fibers
- Rapid or more severe inflammation leads to:
- Loss of smooth muscle cells
- Medial weakening
- Vascular dilatation
- Aneurysm formation
References
- ↑ Inder SJ, Bobryshev YV, Cherian SM, Wang AY, Lord RS, Masuda K, Yutani C (March 2000). "Immunophenotypic analysis of the aortic wall in Takayasu's arteritis: involvement of lymphocytes, dendritic cells and granulocytes in immuno-inflammatory reactions". Cardiovasc Surg. 8 (2): 141–8. PMID 10737351.
- ↑ Lupi-Herrera E, Sánchez-Torres G, Marcushamer J, Mispireta J, Horwitz S, Vela JE (January 1977). "Takayasu's arteritis. Clinical study of 107 cases". Am. Heart J. 93 (1): 94–103. PMID 12655.
- ↑ Salazar M, Varela A, Ramirez LA, Uribe O, Vasquez G, Egea E, Yunis EJ, Iglesias-Gamarra A (August 2000). "Association of HLA-DRB1*1602 and DRB1*1001 with Takayasu arteritis in Colombian mestizos as markers of Amerindian ancestry". Int. J. Cardiol. 75 Suppl 1: S113–6. PMID 10980348.
- ↑ Seko Y, Takahashi N, Tada Y, Yagita H, Okumura K, Nagai R (August 2000). "Restricted usage of T-cell receptor Vgamma-Vdelta genes and expression of costimulatory molecules in Takayasu's arteritis". Int. J. Cardiol. 75 Suppl 1: S77–83, discussion S85–7. PMID 10980341.
- ↑ 5.0 5.1 Gravanis MB (August 2000). "Giant cell arteritis and Takayasu aortitis: morphologic, pathogenetic and etiologic factors". Int. J. Cardiol. 75 Suppl 1: S21–33, discussion S35–6. PMID 10980333.