Siltuximab: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
|drugClass=[[monoclonal antibody]] | |drugClass=[[monoclonal antibody]] | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=patients with [[ | |indication=patients with multi centric [[Castleman's disease]] ([[MCD]]) who are [[human immunodeficiency virus]] ([[HIV]]) negative and [[human herpesvirus-8]] ([[HHV-8]]) negative. SYLVANT was not studied in patients with MCD who are [[HIV]] positive or [[HHV-8]] positive because SYLVANT did not bind to virally produced [[IL-6]] in a nonclinical study | ||
|adverseReactions=[[pruritus]], [[increased weight]], [[rash]], [[hyperuricemia]], and [[upper respiratory tract infection]] | |adverseReactions=[[pruritus]], [[increased weight]], [[rash]], [[hyperuricemia]], and [[upper respiratory tract infection]] | ||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | ||
| Line 43: | Line 43: | ||
====Long Term Exposure==== | ====Long Term Exposure==== | ||
The safety of long term administration of SYLVANT to patients with MCD was evaluated in Study 3. Study 3 enrolled patients from the initial dose finding study of SYLVANT with MCD who were benefiting from chronic SYLVANT therapy. SYLVANT was administered at a dose of 11 mg/kg every 3 to 6 weeks. At the time of data cut off 19 patients were enrolled. The median age was 44 years (range 18 – 76), 63% male, 84% Caucasian, 11% Asian, and 5% Black. The median exposure to SYLVANT for these 19 patients was 5.1 years (range 3.4 to 7.2). The most common adverse reaction (>20%) reported by subjects receiving SYLVANT in this study was upper respiratory tract infection (63%); diarrhea (32%); pain in extremities, arthralgia and fatigue (21% each). No patient was removed from therapy for any reason. There were no deaths. There were no cumulative toxicities identified with prolonged treatment with SYLVANT. | *The safety of long term administration of SYLVANT to patients with MCD was evaluated in Study 3. Study 3 enrolled patients from the initial dose finding study of SYLVANT with MCD who were benefiting from chronic SYLVANT therapy. SYLVANT was administered at a dose of 11 mg/kg every 3 to 6 weeks. At the time of data cut off 19 patients were enrolled. The median age was 44 years (range 18 – 76), 63% male, 84% Caucasian, 11% Asian, and 5% Black. The median exposure to SYLVANT for these 19 patients was 5.1 years (range 3.4 to 7.2). The most common adverse reaction (>20%) reported by subjects receiving SYLVANT in this study was [[upper respiratory tract infection]] (63%); [[diarrhea]] (32%); pain in extremities, [[arthralgia]] and [[fatigue]] (21% each). No patient was removed from therapy for any reason. There were no deaths. There were no cumulative toxicities identified with prolonged treatment with SYLVANT. | ||
Anaphylaxis and Infusion Related Reactions | ====Anaphylaxis and Infusion Related Reactions==== | ||
*Approximately 750 patients have been treated with SYLVANT. Of these, one patient experienced an [[anaphylactic reaction]]. Data from 249 patients treated with SYLVANT monotherapy forms the basis of the safety evaluation of infusion related reactions. Infusion related reactions were reported in 4.8% of these patients. Symptoms of infusion reactions consisted of [[back pain]], [[chest pain]] or discomfort, [[nausea and vomiting]], [[flushing]], [[erythema]], and [[palpitations]]. | |||
====Immunogenicity==== | |||
*Immunogenicity data are highly dependent on the sensitivity and specificity of the test methods used. Additionally, the observed incidence of a positive result in a test method may be influenced by several factors, including sample handling, timing of sample collection, drug interference, concomitant medication and the underlying disease. Therefore, comparison of the incidence of antibodies to SYLVANT with the incidence of antibodies to other products may be misleading. The clinical significance of anti-siltuximab antibodies following treatment with SYLVANT is not known. | |||
*The immunogenicity of siltuximab has been evaluated using antigen-bridging enzyme immunoassay (EIA) and electrochemiluminescence-based immunoassay (ECLIA) methods. A total of 411 patients across the clinical studies were evaluated at multiple time points for anti-therapeutic antibody (ATA) responses to siltuximab after treatment with SYLVANT. Following SYLVANT dosing, 0.2% (1/411) of patients tested positive for anti-siltuximab antibodies. Further immunogenicity analyses of the single positive sample revealed a low titer of anti-siltuximab antibodies with non-neutralizing capabilities. | |||
*No evidence of altered toxicity profile was identified in the patient who developed antibodies to siltuximab. | |||
|drugInteractions=<i>No in vitro or in vivo drug-drug interaction studies have been conducted with SYLVANT.</i> | |||
====Cytochrome P450 Substrates==== | |||
*[[Cytochrome P450]]s in the liver are down regulated by infection and inflammation stimuli including cytokines such as [[IL-6]]. Inhibition of [[IL-6]] signaling in patients treated with SYLVANT may restore [[CYP450]] activities to higher levels leading to increased metabolism of drugs that are [[CYP450]] substrates compared to metabolism prior to treatment with SYLVANT. | |||
*Upon initiation or discontinuation of SYLVANT, in patients being treated with [[CYP450]] substrates with a narrow therapeutic index, perform therapeutic monitoring of effect (e.g., [[warfarin]]) or drug concentration (e.g., [[cyclosporine]] or [[theophylline]]) as needed and adjust dose. The effect of SYLVANT on [[CYP450]] enzyme activity can persist for several weeks after stopping therapy. Exercise caution when SYLVANT is co-administered with [[CYP3A4]] substrate drugs where a decrease in effectiveness would be undesirable (e.g., [[oral contraceptives]], [[lovastatin]], [[atorvastatin]]). | |||
|FDAPregCat=C | |||
|useInPregnancyFDA=====Risk-Summary==== | |||
*There are no adequate or well-controlled studies in pregnant women. In animal reproduction studies, administration of a human antibody to IL-6 to pregnant cynomolgus monkeys caused decreases in globulin levels in pregnant animals and in the offspring. Siltuximab crossed the placenta in monkeys. Infants born to pregnant women treated with SYLVANT may be at increased risk of infection, and caution is advised in the administration of live vaccines to these infants. SYLVANT should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Advise patients of childbearing potential to avoid pregnancy. Women of childbearing potential should use contraception during and for 3 months after treatment. | |||
====Animal Data==== | |||
*In an embryo-fetal development study, siltuximab doses of 9.2 or 46 mg/kg/week were administered intravenously to pregnant monkeys during gestation days (GD) 20 to 118, which includes the period of organogenesis. Fetuses were evaluated on GD 140, approximately 25 days prior to the natural birth. Exposures at the low and high dose after the 25th administration were approximately 3 and 7 times respectively the exposure in humans at the recommended dose of 11 mg/kg. There was no siltuximab-related maternal or fetal toxicity. However, siltuximab crossed the placenta at both doses and when measured on GD 140, fetal serum concentrations of siltuximab were similar to maternal concentrations. In a combined embryofetal and pre- and post-natal development study, cynomolgus monkeys were intravenously administered doses of 10 or 50 mg/kg/week of a human antibody to IL-6 from GD 20 to natural delivery (GD 167). The offspring was evaluated up to 7 months after birth for developmental effects. No maternal or infant toxicity was observed; however, globulin levels were decreased in pregnant animals (GD 34 through lactation day 30) and in the offspring (lactation days 30–120) at both doses. | |||
|useInNursing=*It is not known whether siltuximab is excreted in human milk or absorbed systemically after ingestion. Because many drugs and [[immunoglobulins]] are excreted in human milk, and because of the potential for adverse reactions in nursing infants from SYLVANT, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. | |||
|useInPed=The safety and efficacy of SYLVANT have not been established in pediatric patients. | |||
|useInGeri=*Of the patients treated with SYLVANT monotherapy in clinical studies 127 (35%) were 65 years and older. No overall differences in safety profile were observed between these patients and younger patients, and other reported clinical experience has not identified differences in the safety profile between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. Clinical studies did not include sufficient numbers of patients aged 65 years and older to determine the effect of age on efficacy in MCD population. | |||
|useInRenalImpair=*Based on a population pharmacokinetic analysis using data from clinical trials in patients, no significant difference in siltuximab clearance was observed in patients with pre-existing [[renal impairment]] ([[creatinine clearance]] ([[CLCr]]) ≥15 mL/min) compared to patients with baseline normal [[renal function]] ([[CLCr]] ≥90 mL/min). No initial dosage adjustment is necessary for patients with [[CLCr]] ≥15 mL/min. The potential effect of end stage renal disease on siltuximab pharmacokinetics cannot be determined | |||
|useInHepaticImpair=*Based on a population pharmacokinetic analysis using data from clinical trials in patients, no significant difference in siltuximab clearance was observed in patients with pre-existing mild to moderate hepatic impairment ([[Child-Pugh]] Class A and B, respectively) compared to patients with baseline normal hepatic function. No initial dosage adjustment is necessary for patients with mild to moderate [[hepatic impairment]]. Patients with baseline severe [[hepatic impairment]] ([[Child-Pugh]] Class C) were not included in clinical trials. | |||
|overdose=No case of overdose has been reported. | |||
|drugBox={{Drugbox | |||
| Verifiedfields = changed | |||
| Watchedfields = changed | |||
| verifiedrevid = 464390611 | |||
| image = | |||
<!--Monoclonal antibody data--> | |||
| type = mab | |||
| mab_type = mab | |||
| source = xi/o | |||
| target = [[Interleukin-6|IL-6]] | |||
<!--Clinical data--> | |||
| tradename = Sylvant | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = C | |||
| pregnancy_category = | |||
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled--> | |||
| legal_CA = <!-- Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| legal_UK = <!-- GSL, P, POM, CD, or Class A, B, C --> | |||
| legal_US = Rx-only | |||
| legal_status = | |||
| routes_of_administration = | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = | |||
| elimination_half-life = | |||
| excretion = | |||
<!--Identifiers--> | |||
| CAS_number_Ref = {{cascite|changed|??}} | |||
| CAS_number = 541502-14-1 | |||
| ATC_prefix = L04 | |||
| ATC_suffix = AC11 | |||
| synonyms = CNTO 328 | |||
| PubChem = | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = T4H8FMA7IM | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = NA | |||
<!--Chemical data--> | |||
| C=6450 | H=9932 | N=1688 | O=2016 | S=50 | |||
| molecular_weight = 145.0 [[kDa]] | |||
}} | |||
|mechAction=*Siltuximab binds human [[IL-6]] and prevents the binding of [[IL-6]] to both soluble and membrane-bound [[IL-6]] receptors. [[IL-6]] has been shown to be involved in diverse normal physiologic processes such as induction of [[immunoglobulin secretion]]. Overproduction of [[IL-6]] has been linked to systemic manifestations in patients with [[MCD]]. | |||
|PD=*'''Cardiac Electrophysiology''': The effect of multiple doses of SYLVANT (15 mg/kg every 3 weeks for 4 cycles) on the QTc interval was evaluated in an open label, single arm study in 30 patients with Monoclonal Gammopathy of Undetermined Significance, Smoldering Multiple Myeloma, or Indolent Multiple Myeloma. No large changes in the mean QTc interval (i.e., >20 ms) were detected in the study. | |||
*Measurement of IL-6 concentrations in serum or plasma during treatment should not be used as pharmacodynamic marker, as siltuximab-neutralized antibody-IL-6 complexes interfere with current immunological-based IL-6 quantification methods. | |||
|alcohol=Alcohol-Siltuximab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Siltuximab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 14:08, 16 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Siltuximab is a monoclonal antibody that is FDA approved for the treatment of patients with multi centric Castleman's disease (MCD) who are human immunodeficiency virus (HIV) negative and human herpesvirus-8 (HHV-8) negative. SYLVANT was not studied in patients with MCD who are HIV positive or HHV-8 positive because SYLVANT did not bind to virally produced IL-6 in a nonclinical study. Common adverse reactions include pruritus, increased weight, rash, hyperuricemia, and upper respiratory tract infection.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Siltuximab FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Siltuximab in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Siltuximab in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Siltuximab FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Siltuximab in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Siltuximab in pediatric patients.
Contraindications
- Severe hypersensitivity reaction to siltuximab or any of the excipients in SYLVANT.
Warnings
Concurrent Active Severe Infections
- Do not administer SYLVANT to patients with severe infections until the infection resolves. SYLVANT may mask signs and symptoms of acute inflammation including suppression of fever and of acute phase reactants such as C-reactive protein (CRP). Monitor patients receiving SYLVANT closely for infections. Institute prompt anti-infective therapy and do not administer further SYLVANT until the infection resolves.
Vaccinations
- Do not administer live vaccines to patients receiving SYLVANT because IL-6 inhibition may interfere with the normal immune response to new antigens.
Infusion Related Reactions and Hypersensitivity
- Stop the infusion of SYLVANT if the patient develops signs of anaphylaxis. Discontinue further therapy with SYLVANT.
- Stop the infusion if the patient develops a mild to moderate infusion reaction. If the reaction resolves, the SYLVANT infusion may be restarted at a lower infusion rate. Consider medication with antihistamines, acetaminophen, and corticosteroids. Discontinue SYLVANT if the patient does not tolerate the infusion following these interventions.
- Administer SYLVANT in a setting that provides resuscitation equipment, medication, and personnel trained to provide resuscitation.
Gastrointestinal Perforation
- Gastrointestinal perforation has been reported in clinical trials although not in MCD trials. Use with caution in patients who may be at increased risk for GI perforation. Promptly evaluate patients presenting with symptoms that may be associated or suggestive of GI perforation.
Adverse Reactions
Clinical Trials Experience
The following adverse reactions are also discussed in other sections of the labeling:
- Concurrent active severe infections.
- Infusion-related reactions and hypersensitivity.
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- The most common adverse reactions (>10% compared to placebo) during treatment with SYLVANT in the MCD clinical trial were pruritus, increased weight, rash, hyperuricemia, and upper respiratory tract infection.
- The data presented below in TABLE 3 were collected from Study 1. Study 1, in MCD, was an international, multicenter, randomized phase 2 study of every 3 week infusions comparing SYLVANT and best supportive care (BSC) to placebo and BSC. There were 53 patients randomized to the SYLVANT arm at a dose of 11 mg/kg and 26 patients randomized to the placebo arm. Of the 26 placebo-treated patients, 13 patients subsequently crossed-over to receive SYLVANT. The median age was 48 years (range 20 to 78), 66% male, 48% Asian, 39% White, 4% Black or African American, 7% other. The patients randomized to SYLVANT received a median of 19 infusions (range 1 to 50) compared to patients randomized to placebo who received a median of 8 infusions (range 2 to 32). To control for disparate exposure between arms, Table 3 reports the per patient incidence of adverse reactions that occurred during the first 8 infusions. Adverse reactions that occurred >3% in the SYLVANT arm are presented.
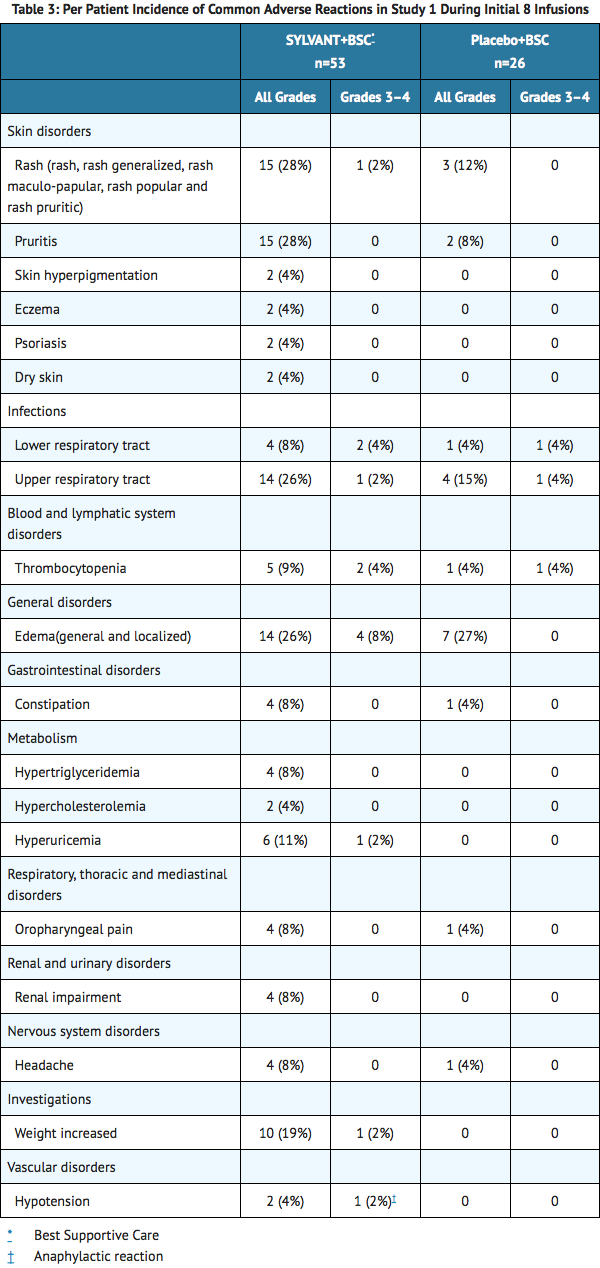
- SYLVANT was also evaluated as a single agent in another hematologic disease in Study 2. Study 2 was an international, multicenter, randomized phase 2 study of every 4 week infusions comparing SYLVANT and BSC to placebo and BSC. There were 50 patients randomized to the SYLVANT arm at a dose of 15 mg/kg (unapproved dose) and 26 patients randomized to the placebo arm. The median age was 72 years (range 50 to 85), 58% male, 96% White, 1% Asian, 1% Black, and 1% other. The median number of infusions in both arms was 3 (range 1 to 4). The study was stopped early due to a lack of efficacy. Adverse reactions that occurred >3% in the SYLVANT arm are presented in Table 4.
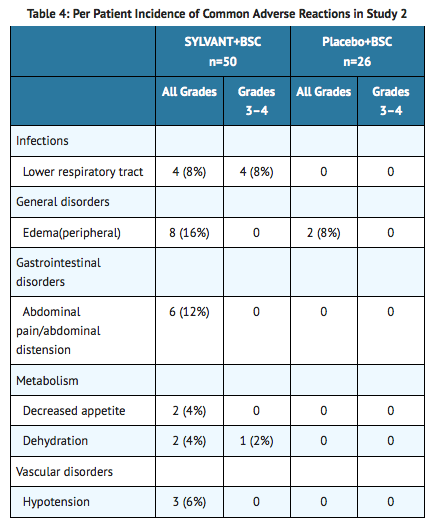
Long Term Exposure
- The safety of long term administration of SYLVANT to patients with MCD was evaluated in Study 3. Study 3 enrolled patients from the initial dose finding study of SYLVANT with MCD who were benefiting from chronic SYLVANT therapy. SYLVANT was administered at a dose of 11 mg/kg every 3 to 6 weeks. At the time of data cut off 19 patients were enrolled. The median age was 44 years (range 18 – 76), 63% male, 84% Caucasian, 11% Asian, and 5% Black. The median exposure to SYLVANT for these 19 patients was 5.1 years (range 3.4 to 7.2). The most common adverse reaction (>20%) reported by subjects receiving SYLVANT in this study was upper respiratory tract infection (63%); diarrhea (32%); pain in extremities, arthralgia and fatigue (21% each). No patient was removed from therapy for any reason. There were no deaths. There were no cumulative toxicities identified with prolonged treatment with SYLVANT.
Anaphylaxis and Infusion Related Reactions
- Approximately 750 patients have been treated with SYLVANT. Of these, one patient experienced an anaphylactic reaction. Data from 249 patients treated with SYLVANT monotherapy forms the basis of the safety evaluation of infusion related reactions. Infusion related reactions were reported in 4.8% of these patients. Symptoms of infusion reactions consisted of back pain, chest pain or discomfort, nausea and vomiting, flushing, erythema, and palpitations.
Immunogenicity
- Immunogenicity data are highly dependent on the sensitivity and specificity of the test methods used. Additionally, the observed incidence of a positive result in a test method may be influenced by several factors, including sample handling, timing of sample collection, drug interference, concomitant medication and the underlying disease. Therefore, comparison of the incidence of antibodies to SYLVANT with the incidence of antibodies to other products may be misleading. The clinical significance of anti-siltuximab antibodies following treatment with SYLVANT is not known.
- The immunogenicity of siltuximab has been evaluated using antigen-bridging enzyme immunoassay (EIA) and electrochemiluminescence-based immunoassay (ECLIA) methods. A total of 411 patients across the clinical studies were evaluated at multiple time points for anti-therapeutic antibody (ATA) responses to siltuximab after treatment with SYLVANT. Following SYLVANT dosing, 0.2% (1/411) of patients tested positive for anti-siltuximab antibodies. Further immunogenicity analyses of the single positive sample revealed a low titer of anti-siltuximab antibodies with non-neutralizing capabilities.
- No evidence of altered toxicity profile was identified in the patient who developed antibodies to siltuximab.
Postmarketing Experience
There is limited information regarding Siltuximab Postmarketing Experience in the drug label.
Drug Interactions
No in vitro or in vivo drug-drug interaction studies have been conducted with SYLVANT.
Cytochrome P450 Substrates
- Cytochrome P450s in the liver are down regulated by infection and inflammation stimuli including cytokines such as IL-6. Inhibition of IL-6 signaling in patients treated with SYLVANT may restore CYP450 activities to higher levels leading to increased metabolism of drugs that are CYP450 substrates compared to metabolism prior to treatment with SYLVANT.
- Upon initiation or discontinuation of SYLVANT, in patients being treated with CYP450 substrates with a narrow therapeutic index, perform therapeutic monitoring of effect (e.g., warfarin) or drug concentration (e.g., cyclosporine or theophylline) as needed and adjust dose. The effect of SYLVANT on CYP450 enzyme activity can persist for several weeks after stopping therapy. Exercise caution when SYLVANT is co-administered with CYP3A4 substrate drugs where a decrease in effectiveness would be undesirable (e.g., oral contraceptives, lovastatin, atorvastatin).
Use in Specific Populations
Pregnancy
Risk-Summary
- There are no adequate or well-controlled studies in pregnant women. In animal reproduction studies, administration of a human antibody to IL-6 to pregnant cynomolgus monkeys caused decreases in globulin levels in pregnant animals and in the offspring. Siltuximab crossed the placenta in monkeys. Infants born to pregnant women treated with SYLVANT may be at increased risk of infection, and caution is advised in the administration of live vaccines to these infants. SYLVANT should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Advise patients of childbearing potential to avoid pregnancy. Women of childbearing potential should use contraception during and for 3 months after treatment.
Animal Data
- In an embryo-fetal development study, siltuximab doses of 9.2 or 46 mg/kg/week were administered intravenously to pregnant monkeys during gestation days (GD) 20 to 118, which includes the period of organogenesis. Fetuses were evaluated on GD 140, approximately 25 days prior to the natural birth. Exposures at the low and high dose after the 25th administration were approximately 3 and 7 times respectively the exposure in humans at the recommended dose of 11 mg/kg. There was no siltuximab-related maternal or fetal toxicity. However, siltuximab crossed the placenta at both doses and when measured on GD 140, fetal serum concentrations of siltuximab were similar to maternal concentrations. In a combined embryofetal and pre- and post-natal development study, cynomolgus monkeys were intravenously administered doses of 10 or 50 mg/kg/week of a human antibody to IL-6 from GD 20 to natural delivery (GD 167). The offspring was evaluated up to 7 months after birth for developmental effects. No maternal or infant toxicity was observed; however, globulin levels were decreased in pregnant animals (GD 34 through lactation day 30) and in the offspring (lactation days 30–120) at both doses.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Siltuximab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Siltuximab during labor and delivery.
Nursing Mothers
- It is not known whether siltuximab is excreted in human milk or absorbed systemically after ingestion. Because many drugs and immunoglobulins are excreted in human milk, and because of the potential for adverse reactions in nursing infants from SYLVANT, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
The safety and efficacy of SYLVANT have not been established in pediatric patients.
Geriatic Use
- Of the patients treated with SYLVANT monotherapy in clinical studies 127 (35%) were 65 years and older. No overall differences in safety profile were observed between these patients and younger patients, and other reported clinical experience has not identified differences in the safety profile between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. Clinical studies did not include sufficient numbers of patients aged 65 years and older to determine the effect of age on efficacy in MCD population.
Gender
There is no FDA guidance on the use of Siltuximab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Siltuximab with respect to specific racial populations.
Renal Impairment
- Based on a population pharmacokinetic analysis using data from clinical trials in patients, no significant difference in siltuximab clearance was observed in patients with pre-existing renal impairment (creatinine clearance (CLCr) ≥15 mL/min) compared to patients with baseline normal renal function (CLCr ≥90 mL/min). No initial dosage adjustment is necessary for patients with CLCr ≥15 mL/min. The potential effect of end stage renal disease on siltuximab pharmacokinetics cannot be determined
Hepatic Impairment
- Based on a population pharmacokinetic analysis using data from clinical trials in patients, no significant difference in siltuximab clearance was observed in patients with pre-existing mild to moderate hepatic impairment (Child-Pugh Class A and B, respectively) compared to patients with baseline normal hepatic function. No initial dosage adjustment is necessary for patients with mild to moderate hepatic impairment. Patients with baseline severe hepatic impairment (Child-Pugh Class C) were not included in clinical trials.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Siltuximab in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Siltuximab in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Siltuximab Administration in the drug label.
Monitoring
There is limited information regarding Siltuximab Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Siltuximab and IV administrations.
Overdosage
No case of overdose has been reported.
Pharmacology
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Template:Infobox drug/mab source |
| Target | IL-6 |
| Clinical data | |
| Trade names | Sylvant |
| Synonyms | CNTO 328 |
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| ChemSpider | |
| UNII | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C6450H9932N1688O2016S50 |
| Molar mass | 145.0 kDa |
| | |
Mechanism of Action
- Siltuximab binds human IL-6 and prevents the binding of IL-6 to both soluble and membrane-bound IL-6 receptors. IL-6 has been shown to be involved in diverse normal physiologic processes such as induction of immunoglobulin secretion. Overproduction of IL-6 has been linked to systemic manifestations in patients with MCD.
Structure
There is limited information regarding Siltuximab Structure in the drug label.
Pharmacodynamics
- Cardiac Electrophysiology: The effect of multiple doses of SYLVANT (15 mg/kg every 3 weeks for 4 cycles) on the QTc interval was evaluated in an open label, single arm study in 30 patients with Monoclonal Gammopathy of Undetermined Significance, Smoldering Multiple Myeloma, or Indolent Multiple Myeloma. No large changes in the mean QTc interval (i.e., >20 ms) were detected in the study.
- Measurement of IL-6 concentrations in serum or plasma during treatment should not be used as pharmacodynamic marker, as siltuximab-neutralized antibody-IL-6 complexes interfere with current immunological-based IL-6 quantification methods.
Pharmacokinetics
There is limited information regarding Siltuximab Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Siltuximab Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Siltuximab Clinical Studies in the drug label.
How Supplied
There is limited information regarding Siltuximab How Supplied in the drug label.
Storage
There is limited information regarding Siltuximab Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Siltuximab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Siltuximab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Siltuximab Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Siltuximab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Siltuximab Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Siltuximab Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- Pages with script errors
- Articles with changed CASNo identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Infobox drug tracked parameters
- Drugs that are a monoclonal antibody