Sandbox: differentialdx maria: Difference between revisions
No edit summary |
No edit summary |
||
| (21 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{|style="border: 5px; font-size: 90%; margin: 5px; width: 1000px" align=center | |||
!style="padding: 5px 5px; background: #4479BA; font-weight: bold; text-align:center;" colspan="2"|{{fontcolor|#FFF|'''Genetic lesions and potential therapeutic drugs'''<br><SMALL> Adapted from Chiaretti et al</SMALL>}} | |||
|valign=top| | |||
|+ | |||
|- | |||
!style="background: #4479BA; width: 300px; text-align:center;" | {{fontcolor|#FFF|'''Genes involved'''}} | |||
!style="background: #4479BA; width: 300px; text-align:center;" | {{fontcolor|#FFF|'''Therapeutic compound'''}} | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| TAL1 | |||
| style="padding: 5px 5px; background: #F5F5F5;"| HDAC inhibitors | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Notch1 | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Y-secretase inhibitors | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| ABL1 | |||
| style="padding: 5px 5px; background: #F5F5F5;"| ABL1 kinase inhibitors | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| JAK2 | |||
| style="padding: 5px 5px; background: #F5F5F5;"| JAK2 inhibitors | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| JAK1 | |||
| style="padding: 5px 5px; background: #F5F5F5;"| Tyrosine kinase inhibitors | |||
|} | |||
{|style="border: 5px; font-size: 90%; margin: 5px; width: 800px" align=center | |||
!style="padding: 5px 5px; background: #4479BA; font-weight: bold; text-align:center;" colspan="2"|{{fontcolor|#FFF|'''WHO histological classification<br>Tumors of the appendix <br><SMALL> Adapted from WHO/IARC </SMALL>'''}} | |||
|valign=top| | |||
|+ | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Epithelial tumors | |||
|- | |||
| | |||
*Adenoma | |||
:*Tubular | |||
:*Villous | |||
:*Tubulovillous | |||
:*Serrated | |||
*Carcinoma | |||
:*Adenocarcinoma | |||
:*Mucinous adenocarcinoma | |||
:*Signet-ring cell carcinoma | |||
:*Small cell carcinoma | |||
:*Undifferentiated carcinoma | |||
*Carcinoid (well differentiated endocrine neoplasm) | |||
*Tubular carcinoid | |||
*Goblet cell carcinoid (mucinous carcinoid) | |||
*Mixed carcinoid-adenocarcinoma | |||
*Others | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" | Non-epithelial tumors | |||
|- | |||
| | |||
*Neuroma | |||
*Lipoma | |||
*Leiomyoma | |||
*Gastrointestinal stromal tumor | |||
*Leiomyosarcoma | |||
*Kaposi sarcoma | |||
*Others | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Secondary tumors | |||
|- | |||
| | |||
*Metastasis (eg. Primary of urogenital tract, breast, lung) | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Hyperplastic polyp | |||
|} | |||
CIN is classified in grades: | |||
{| class="wikitable" | |||
|- | |||
! Histology Grade | |||
! Description | |||
! Image | |||
|- | |||
| – | |||
| Normal cervical epithelium | |||
| [[Image:Cervical_intraepithelial_neoplasia_(1)_normal_squamous_epithelium.jpg|center|100px]] | |||
|- | |||
| '''CIN 1''' (Grade I) | |||
| The least risky type, represents only mild [[dysplasia]], or abnormal cell growth.It is confined to the basal 1/3 of the epithelium. This corresponds to infection with HPV, and typically will be cleared by immune response in a year or so, though can take several years to clear. | |||
| [[Image:Cervical_intraepithelial_neoplasia_(2)_koilocytosis.jpg|center|100px]] | |||
|- | |||
| '''CIN 2/3''' | |||
| Formerly subdivided into CIN2 and CIN3. | |||
|- | |||
| '''CIN 2''' (Grade II) | |||
| Moderate dysplasia confined to the basal 2/3 of the epithelium | |||
| [[Image:100px-Cervical_intraepithelial_neoplasia_(3)_CIN2.jpg|center|100px]] | |||
|- | |||
| '''CIN 3''' (Grade III) | |||
| Severe dysplasia that spans more than 2/3 of the epithelium, and may involve the full thickness. This lesion may sometimes also be referred to as cervical [[carcinoma in situ]]. | |||
| [[Image:451px-Cervical_intraepithelial_neoplasia_(4)_CIN3.jpg|center|100px]] | |||
|} | |||
{|style="border: 5px; font-size: 90%; margin: 5px; width: 1000px" align=center | |||
!style="padding: 5px 5px; background: #4479BA; font-weight: bold; text-align:center;" colspan="2"|{{fontcolor|#FFF|'''Ultrasound findings of common liver masses'''}} | |||
|valign=top| | |||
|+ | |||
!style="background: #4479BA; width: 300px; text-align:center;" | {{fontcolor|#FFF|'''Common liver masses'''}} | |||
!style="background: #4479BA; width: 300px; text-align:center;" | {{fontcolor|#FFF|'''Ultrasound finding'''}} | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" | Hepatic hemangioma | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Well-demarcated | |||
*Homogeneous | |||
*Hyperechoic mass | |||
*May be hypoechoic in patients with fatty infiltration | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" | Focal nodular hyperplasia | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Detectable lesions | |||
*Central scar with displacement of peripheral vasculature (Doppler examination) | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" | Hepatic adenoma | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Large | |||
*Right lobe of the liver | |||
*Central hypoechoic region | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" | Idiopathic noncirrhotic portal hypertension | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Isoechoic lesions | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" | Hepatocellular carcinoma | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Poorly-defined margins | |||
*Coarse, irregular internal echoes | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" | Cholangiocarcinoma | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Hypo-, iso-, or hyperechoic | |||
*Homogenous or heterogenous | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" | Metastases | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Metastases from adenocarcinoma | |||
:*Multiple and hypoechoic in comparison with the surrounding liver parenchyma | |||
|} | |||
{|style="border: 5px; font-size: 90%; margin: 5px; width: 1000px" align=center | |||
!style="padding: 5px 5px; background: #4479BA; font-weight: bold; text-align:center;" colspan="2"|{{fontcolor|#FFF|'''Classification of liver mass'''}} | |||
|valign=top| | |||
|+ | |||
|- | |||
| style="background: #4479BA; width: 300px; text-align:center;" | {{fontcolor|#FFF|'''Benign'''}} | |||
| style="background: #4479BA; width: 300px; text-align:center;" | {{fontcolor|#FFF|'''Malignant'''}} | |||
|- | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Hepatic hemangioma | |||
*Focal nodular hyperplasia | |||
*Hepatic adenoma | |||
*Idiopathic noncirrhotic portal hypertension | |||
*Nodular regenerative hyperplasia | |||
*Regenerative nodules | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Hepatocellular carcinoma | |||
*Cholangiocarcinoma | |||
*Metastatic disease | |||
|} | |||
{| style="border: 0px; font-size: 90%; margin: 3px; width: 1000px" align=center | |||
|valign=top| | |||
|+ | |||
! style="background: #4479BA; width: 100px;" | {{fontcolor|#FFF|'''Cavitating causes'''}} | |||
! style="background: #4479BA; width: 300px;" | {{fontcolor|#FFF|'''Conditions'''}} | |||
! style="background: #4479BA; width: 300px;" | {{fontcolor|#FFF|'''Description'''}} | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" | Malignancy | |||
| style="padding: 5px 5px; background: #F5F5F5;"| | |||
Cancer | |||
*Primary bronchogenic carcinoma(especially squamous cell carcinoma) | |||
*Cavitating pulmonary metastases (especially squamous cell carcinoma, GI adenocarincoma, sarcoma) | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
Cancer | |||
*Thick wall | |||
*Irregular shape | |||
*Disort of adjacent structures | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" | Infection | |||
| style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Pulmonary bacterial abscess/cavitating pneumonia | |||
*Empyema | |||
*Post-pneumonic pneumatocoele | |||
*Septic pulmonary emboli | |||
*Pulmonary coccidioidomycosis | |||
*Pulmonary actinomycosis / thoracic actinomycosis | |||
*Pulmonary nocardiosis | |||
*Melioidosis | |||
*Pulmonary cryptococcosis | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
Abscess: | |||
*Round in all projections | |||
*Abruptly interrupts bronchovascular structures | |||
*May form a acute angle with the costal surface / chest wall | |||
*Abscesses have thick irregular walls | |||
*Abscesses usually have an acute angle (claw sign) | |||
Empyema: | |||
*Smoother margins | |||
*Lentiform shape | |||
*Distort and compresses adjacent lung | |||
*Empyemas have obtuse angles | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" | | |||
Non-infectious | |||
| style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Granulomatosis with polyangitis | |||
*Rheumatoid nodules | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*May be single or multiple | |||
*Size ranges from 0.5-7 cm 3,5 | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" | | |||
Vascular | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Pulmonary infarct | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Consolidation with internal air lucencies, | |||
*"Bubbly consolidation"; this represent non-infarcted aerated lung parenchyma | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" | | |||
Trauma | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Pneumatocoeles | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Smooth inner margins | |||
*Contain little if any fluid | |||
*Wall (if visible) is thin and regular | |||
*Persist despite absence of symtpoms | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" | Congenital | |||
| style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Congenital cystic adenomatoid malformation (CCAM) | |||
*Pulmonary sequestration | |||
*Bronchogenic cyst | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Radiological features vary according to disease | |||
*To learn more about congenital lung cavitations, click in the blue links. | |||
|} | |||
{|style="border: 5px; font-size: 90%; margin: 5px; width: 1000px" align=center | |||
!style="padding: 5px 5px; background: #4479BA; font-weight: bold; text-align:center;" colspan="2"|{{fontcolor|#FFF|'''Imaging features of lung mass'''}} | |||
|valign=top| | |||
|+ | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" | Hyperdense pulmonary mass | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" | Cavitating pulmonary mass | |||
|- | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Granuloma (most common) | |||
*Pulmonary hamartoma | |||
*Bronchogenic carcinoma | |||
*Carcinoid tumours | |||
*Pulmonary metastases: | |||
:*Mucoid calcification of mucinous adenocarcinoma | |||
:*Breast carcinoma | |||
:*Gastrointestinal tract adenocarcinoma | |||
*Dystrophic calcification: | |||
:*Papillary thyroid carcinoma | |||
:*Giant cell tumor of bone | |||
:*Synovial sarcoma | |||
*Treated pulmonary metastases | |||
:*Osteosarcoma | |||
:*Chondrosarcoma | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
'''Cancer''' | |||
:*Bronchogenic carcinoma (most common) | |||
:*Squamous cell carcinoma | |||
'''Autoimmune''' | |||
:*Granulomas (Wegener's granulomatosis) | |||
:*Rheumatoid arthritis | |||
:*Rheumatoid nodules | |||
'''Vascular''' | |||
:*Septic pulmonary embolus | |||
'''Infections (bacterial/fungal)''' | |||
:*Pulmonary abscess | |||
:*Pulmonary tuberculosis | |||
'''Trauma''' | |||
:*Pneumatocoeles | |||
'''Youth''' | |||
:*CPAM | |||
:*Pulmonary sequestration | |||
:*Bronchogenic cyst | |||
|} | |||
{|style="border: 5px; font-size: 90%; margin: 5px; width: 1000px" align=center | |||
!style="padding: 5px 5px; background: #4479BA; font-weight: bold; text-align:center;" colspan="3"|{{fontcolor|#FFF|'''Classification of Benign and Malignant Pulmonary Mass'''}} | |||
|valign=top| | |||
|+ | |||
! style="font-weight: bold;" | Lung mass (location) | |||
! style="font-weight: bold;" | Benign | |||
! style="font-weight: bold;" | Malignant | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Endobronchial | |||
| style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Bronchial atresia | |||
*Bronchial hamartoma | |||
*Bronchogenic cysts | |||
*Pulmonary bacterial abscess | |||
*Bronchial anthracofibrosis | |||
*Allergic bronchopulmonary aspergillosis | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Squamous dysplasia of lung | |||
*Squamous cell lung carcinoma | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Parenchymal | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Granuloma | |||
*Pulmonary hamartoma | |||
*Pulmonary bacterial abscess | |||
*Pulmonary infract septic | |||
*Pulmonary emboli | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Bronchogenic carcinoma | |||
*Carcinoid tumors | |||
*Pulmonary metastases | |||
*Papillary thyroid carcinoma | |||
*Giant cell tumor of bones | |||
*Synovial sarcoma | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" | Pleural | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Pleural effusion | |||
*Empyema | |||
*Hemothorax | |||
*Lipoma | |||
*Splenosis | |||
*Tuberculosis | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Mesothelioma | |||
*Metastasic pleural disease | |||
*Invasive thymoma | |||
*Pleural fibrosarcoma | |||
*Pleural liposarcoma | |||
*Primary pleural lymphoma | |||
*Pleural synovial sarcoma | |||
|- | |||
|} | |||
{|style="border: 5px; font-size: 90%; margin: 5px; width: 1000px" align=center | |||
!style="padding: 5px 5px; background: #4479BA; font-weight: bold; text-align:center;" colspan="3"|{{fontcolor|#FFF|''' Radiologic Features Suggestive of Benign or Malignant Solitary Pulmonary Nodules''' <br><SMALL> Adapted from American Academy of Family Physicians <ref name="CDC"> Solitary Pulmonary Nodule: Morphological Evaluation. http://pubs.rsna.org/doi/pdf/10.1148/radiographics.20.1.g00ja0343 Accessed on March 15, 2016 </ref></SMALL>}} | |||
|valign=top| | |||
|+ | |||
|- | |||
! style="background: #4479BA; width: 300px;" | {{fontcolor|#FFF|'''Radiologic feature'''}} | |||
! style="background: #4479BA; width: 300px;" | {{fontcolor|#FFF|'''Benign'''}} | |||
! style="background: #4479BA; width: 300px;" | {{fontcolor|#FFF|'''Malignant'''}} | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Size | |||
|style="padding: 5px 5px; background: #F5F5F5;"|< 5 mm | |||
|style="padding: 5px 5px; background: #F5F5F5;"|> 10 mm | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Border | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Smooth | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Irregular or spiculated | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Density | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Dense, solid | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Nonsolid, “ground glass” | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Calcification | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Typically a benign feature, especially in “concentric,” “central,” “popcorn-like,” or “homogeneous” patterns | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Typically noncalcified, or “eccentric” calcification | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Doubling time | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Less than one month; more than one year | |||
|style="padding: 5px 5px; background: #F5F5F5;"| One month to one year | |||
|} | |||
===Recommendations for Follow-up and Management of Nodules <8 mm Detected Incidentally at Non-screening CT=== | |||
{| border="2" cellpadding="20" cellspacing="0" | |||
!style="background: #4479BA; width: 300px;" | {{fontcolor|#FFF|Nodule Size (mm)}} | |||
!style="background: #4479BA; width: 300px;" | {{fontcolor|#FFF|'''Low risk''' patients}} | |||
!style="background: #4479BA; width: 300px;" | {{fontcolor|#FFF|'''High risk''' patients}} | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"|Less than or equal to 4 | |||
|style="padding: 5px 5px; background: #F5F5F5;"| |No follow-up needed. | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Follow-up at 12 months. If no change, no further imaging needed. | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"|>4 - 6 | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Follow-up at 12 months. If no change, no further imaging needed. | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Initial follow-up CT at 6 -12 months and then at 18 - 24 months if no change. | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"|>6 - 8 | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Initial follow-up CT at 6 -12 months and then at 18 - 24 months if no change. | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Initial follow-up CT at 3 - 6 months and then at 9 -12 and 24 months if no change. | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"|> 8 | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Follow-up CTs at around 3, 9, and 24 months. Dynamic contrast enhanced CT, PET, and/or biopsy | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Same at for low risk patients | |||
|} | |||
Note: Newly detected indeterminate nodule in persons 35 years of age or older.<ref>Heber MacMahon, John H. M. Austin, Gordon Gamsu, Christian J. Herold, James R. Jett, David P. Naidich, Edward F. Patz, Jr, and Stephen J. Swensen. [http://radiology.rsnajnls.org/cgi/content/abstract/237/2/395 Guidelines for Management of Small Pulmonary Nodules Detected on CT Scans: A Statement from the Fleischner Society.] Radiology 2005 237: 395-400.</ref> | |||
* '''Low risk patients''': Minimal or absent history of smoking and of other known risk factors. | |||
* '''High risk patients''': History of smoking or of other known risk factors. | |||
{|style="border: 5px; font-size: 90%; margin: 5px; width: 1000px" align=center | |||
!style="padding: 5px 5px; background: #4479BA; font-weight: bold; text-align:center;" colspan="2"|{{fontcolor|#FFF|'''Differential Diagnosis for Solitary Pulmonary''' <br><SMALL> Adapted from Erasmus et al. <ref name="CDC"> Solitary Pulmonary Nodule: Morphological Evaluation. http://pubs.rsna.org/doi/pdf/10.1148/radiographics.20.1.g00ja0343 Accessed on March 15, 2016 </ref></SMALL>}} | |||
|valign=top| | |||
|+ | |||
! style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Differential Diagnosis}} | |||
! style="background: #4479BA; width: 300px;" | {{fontcolor|#FFF|Causes}} | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Malignant neoplasms | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Bronchogenic carcinoma | |||
*Carcinoid tumor | |||
*Pulmonary lymphoma | |||
*Pulmonary sarcoma | |||
*Solitary metastases | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Benign neoplasms | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Hamartoma | |||
*Adenoma | |||
*Lipoma | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Infectious inflammatory | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Granuloma (tuberculous/fungal) | |||
*Nocardia infection | |||
*Round pneumonia | |||
*Abscess | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Non-infectious inflammatory | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Rheumatoid arthritis | |||
*Wegener's granulomatosis | |||
*Sarcoidosis | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Vascular | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Arteriovenous malformation | |||
*Infarction | |||
*Hematoma | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Congenital | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Bronchial atresia | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Miscellaneous | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*External object | |||
*Pseudotumor | |||
*Pleural thickening | |||
|} | |||
{| style="border: 0px; font-size: 90%; margin: 3px; width: 1000px" align=center | |||
|valign=top| | |||
|+ | |||
! style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Differential Diagnosis}} | |||
! style="background: #4479BA; width: 300px;" | {{fontcolor|#FFF|Similar Features}} | |||
! style="background: #4479BA; width: 300px;" | {{fontcolor|#FFF|Differentiating Features}} | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"|''' [[Pulmonary tuberculosis]]''' | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Cough, weight loss, fatigue, and dyspnea | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*In pulmonary tuberculosis, differentiating features include: size increase despite optimal medical therapy, patients age is usually younger, hemoptysis is an early feature, and CXR anatomical predilection for upper lobes | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"|''' [[ Lung abscess]]''' | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Non-productive cough, weight loss, and chest pain | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*In lung abscess, differentiating features include: acute or sub-acute onset, CXR anatomical predilection for upper lobes, and usually resolve with antibiotic | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"|'''[[Pneumonia]]''' | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Cough, weight loss, fatigue, and dyspnea | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*In pneumonia, differentiating features include: good response to antibiotics, acute onset, predilection on CXR is consolidation, laboratory markers indicate infection | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| '''[[Pulmonary fungal infection]]''' | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Non-productive cough, weight loss, fatigue, and dyspnea | |||
| style="padding: 5px 5px; background: #F5F5F5;"| | |||
*In pulmonary fungal infection, differentiating features include: CXR findings: air-cresecent sign, no response to antibioitcs, and mimics tuberculosis | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"|'''Other non-small cell lung cancers (adenocarcinoma and squamous cell lung cancer)''' | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Non-productive cough, weight loss, fatigue, and dyspnea | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*In other non-small cell lung cancers , differentiating features include: histopathologica features, such as larger size of the anaplastic cells, a higher cytoplasmic-to-nuclear size ratio, and a lack of "salt-and-pepper" chromatin | |||
|} | |||
{| class="wikitable" | |||
! style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Differential Diagnosis}} | |||
! style="background: #4479BA; width: 300px;" | {{fontcolor|#FFF|Similar Features}} | |||
! style="background: #4479BA; width: 300px;" | {{fontcolor|#FFF|Differentiating Features}} | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"|''' [[Pulmonary tuberculosis]]''' | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Chronic [[cough]], [[weight loss]], [[hemoptysis]], nocturnal diaphoresis, [[dyspnea]] | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*In pulmonary tuberculosis, differentiating features include: increase in diameter despite optimal medical therapy, patients age is usually younger, hemoptisis is an early feature, and CXR anatomical predilection for upper lobes | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"|''' [[Sarcoidosis]]''' | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Chronic [[cough]], [[weight loss]], and [[dyspnea]] | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*In lung abscess, differentiating features include: acute or subacute onset, CXR anatomical predilection for upper lobes, and usually resolve with antibiotic | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"|'''[[Pneumonia]]''' | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Cough, fatigue, and dyspnea | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*In pneumonia, differentiating features include: good response to antibiotics, acute onset, predilection on CXR is consolidation, laboratory markers indicate infection | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| '''[[Pulmonary fungal infection]]''' | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Chronic [[cough]], [[weight loss]], [[hemoptysis]], and [[dyspnea]] | |||
| style="padding: 5px 5px; background: #F5F5F5;"| | |||
*In primary fungal infection, differentiating features include: CXR findings: air-cresecent sign, no response to antibioitcs, and mimcs tuberculosis | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"|'''[[Metastases]]''' | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Chronic [[cough]], [[weight loss]], [[hemoptysis]], and [[dyspnea]] | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*In metastases, differentiating features include: multicentricity, involvement of the contralateral hemitorax, and usually primary cancer is known | |||
|} | |||
{| style="border: 0px; font-size: 90%; margin: 3px; width: 1000px" align=center | |||
|valign=top| | |||
|+ | |||
|- | |||
! style="background: #4479BA; width: 50px;" | {{fontcolor|#FFF|'''Stage (TNM criteria)'''}} | |||
! style="background: #4479BA; width: 100px;" | {{fontcolor|#FFF|'''Standard Treatment Options'''}} | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| '''Occult''' | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
* Surgery | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| '''Stage 0''' | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Surgery | |||
*Endobronchial therapies | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| '''Stages IA and IB''' | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Surgery | |||
*Radiation therapy | |||
*IB, if the tumor is >4cm, surgery and chemotherapy | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"|'''Stages IIA and IIB''' | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Surgery | |||
*Neoadjuvant chemotherapy | |||
*Adjuvant chemotherapy | |||
*Radiation therapy | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| '''Stage IIIA''' | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
'''Resected or resectable disease''' | |||
*Surgery | |||
*Neoadjuvant therapy | |||
*Adjuvant therapy | |||
'''Unresectable disease''' | |||
*Radiation therapy | |||
*Chemoradiation therapy | |||
'''Superior sulcus tumors ''' | |||
*Radiation therapy alone | |||
*Radiation therapy and surgery | |||
*Concurrent chemotherapy with radiation therapy and surgery | |||
*Surgery alone (for selected patients) | |||
'''Tumors that invade the chest wall''' | |||
*Surgery | |||
*Surgery and radiation therapy | |||
*Radiation therapy alone | |||
*Chemotherapy combined with radiation therapy and/or surgery | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| '''Stage IIIB''' | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Sequential or concurrent chemotherapy and radiation therapy | |||
*Chemotherapy followed by surgery (for selected patients) | |||
*Radiation therapy alone | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| '''Stage IV''' | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Cytotoxic combination chemotherapy (first line) | |||
*Combination chemotherapy with bevacizumab or cetuximab | |||
*EGFR tyrosine kinase inhibitors (first line) | |||
*EML4-ALK inhibitors in patients with EML-ALK translocations | |||
*Immune checkpoint inhibition with nivolumab for selected patients with squamous or nonsquamous metastatic | |||
Maintenance therapy following first-line chemotherapy | |||
* Endobronchial laser therapy and/or brachytherapy (for obstructing lesions) | |||
* External-beam radiation therapy (primarily for palliation of local symptomatic tumor growth) | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| '''Recurrent''' | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Radiation therapy (for palliation) | |||
*Chemotherapy or kinase inhibitors alone EGFR inhibitors in patients with/without EGFR mutations | |||
*EML4-ALK inhibitors in patients with EML-ALK translocations | |||
*Surgical resection of isolated cerebral metastasis (for highly selected patients) | |||
*Laser therapy or interstitial radiation therapy (for endobronchial lesions) | |||
*Stereotactic radiation surgery (for highly selected patients) | |||
|} | |||
{| style="border: 0px; font-size: 90%; margin: 3px; width: 1000px" align=center | |||
|valign=top| | |||
|+ | |||
|- | |||
! style="background: #4479BA; width: 50px;" | {{fontcolor|#FFF|Type of tumor }} | |||
! style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Biopsy findings}} | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| [[Lung adenocarcinoma]] | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Nuclear atypia | |||
*Eccentrically placed nuclei | |||
*Abundant cytoplasm - classically with mucin vacuoles | |||
*Often conspicuous [[nucleoli]] | |||
*[[Nuclear pseudoinclusions]] | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| [[Squamous cell lung carcinoma]] | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Central nucleus | |||
*Dense appearing cytoplasm, usu. eosinophilic | |||
*Small nucleolus | |||
*Intracellular bridges - classic | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| [[Large cell lung carcinoma]] | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Large polygonal cells and anaplastic cells | |||
*Solid nests without obvious squamous or glandular differentiation | |||
*Moderately abundant cytoplasm | |||
*Well defined cell borders | |||
*Vesicular nuclei, prominent nucleoli | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Adenosquamous carcinoma | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Substantial amounts of squamous and glandular differentiation | |||
*Positive stains for TTF1 and p63 in squamous component | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Sarcomatoid carcinoma | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Sarcoma-like differentiation | |||
*Spindle cells vary morphologically from epithelioid to strikingly spindled and are arranged in haphazard fascicles or storiform pattern | |||
*Moderate to abundant, dense, eosinophilic cytoplasm | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| [[Carcinoid tumor]] | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Medium sized polygonal cells with lightly eosinophilic cytoplasm | |||
*Low nuclear grade, round to oval finely granular nuclei; may have rosettes or small acinar structures with variable mucin | |||
*Scanty vascular stroma, occasionally amyloid stroma with bone | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| [[Salivary gland tumor]] | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Organized in round and sometimes confluent islands, rich in matrix and with dispersed condrocyte-type cells | |||
|- | |||
|} | |||
{|style="border: 5px; font-size: 90%; margin: 5px; width: 1000px" align=center | |||
!style="padding: 5px 5px; background: #4479BA; font-weight: bold; text-align:center;" colspan="3"|{{fontcolor|#FFF|''Organization Screening Guidelines for Non Small Cell Lung Cancer'' <br><SMALL>Adapted from CDC (2016) <ref name="CDC"> Screening for non-small cell lung cancer. http://www.cdc.gov/cancer/lung/pdf/guidelines.pdf Accessed on February 22, 2016 </ref></SMALL>}} | |||
|valign=top| | |||
|+ | |||
|- | |||
! style="background: #4479BA; width: 300px;" | {{fontcolor|#FFF|Organization}} | |||
! style="background: #4479BA; width: 350px;" | {{fontcolor|#FFF|Groups eligible for screening}} | |||
! style="background: #4479BA; width: 100px;" | {{fontcolor|#FFF|Year}} | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| American Academy of Family Practice | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Evidence is insufficient to recommend for or against screening | |||
|style="padding: 5px 5px; background: #F5F5F5;"| 2013 | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| American Association of Thoracic Surgery | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
1. Age 55 to 79 years with 30 pack year smoking history. | |||
2. Long term lung cancer survivors who have completed 4 years of surveillance without recurrence and who can tolerate lung cancer treatment following screening to detect second primary lung cancer until the age of 79. | |||
3. Age 50 to 79 years with a 20 pack year smoking history and additional comorbidity that produces a cumulative risk of developing lung cancer ≥ 5% in 5 years | |||
| style="padding: 5px 5px; background: #F5F5F5;"| | |||
2012 | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| American Cancer Society | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
Age 55 to 74 years with ≥30 pack year smoking history, who either currently smoke or have quit within the past 15 years, and who are in relatively good health. | |||
| style="padding: 5px 5px; background: #F5F5F5;"| | |||
2015 | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| American College of Chest Physicans | |||
| style="padding: 5px 5px; background: #F5F5F5;"| | |||
Age 55 to 74 years with ≥30 pack year smoking history,who either currently smoke or have quit within the past 15 years | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
2013 | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| American Society of Clinical Oncology | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
Age 55 to 74 years with ≥30 pack year smoking history,who either currently smoke or have quit within the past 15 years | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
2012 | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| American Lung Association | |||
| style="padding: 5px 5px; background: #F5F5F5;"| | |||
Age 55 to 74 years with ≥ 30 pack year smoking history and no history of lung cancer | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
2012 | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Medicaid Services | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
Age 55 to 77 years with ≥ 30 pack year smoking history and smoking cessation < 15 years | |||
| style="padding: 5px 5px; background: #F5F5F5;"| | |||
2015 | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| National Comprehensive Cancer Network | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
Age 55 to74 years with ≥30 packyear smoking history and smoking cessation < 15 years OR Age ≥ 50 years and ≥20 pack year smoking history and additional risk factor (other than secondhand smoke exposure | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
2015 | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| U.S Preventive Services Task Force | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
Age 55 to 80 years with ≥30 pack year smoking history and smoking cessation < 15 years. | |||
| style="padding: 5px 5px; background: #F5F5F5;"| | |||
2013 | |||
|} | |||
{| style="border: 0px; font-size: 90%; margin: 3px; width: 1000px" align=center | |||
|valign=top| | |||
|+ | |||
|- | |||
! style="background: #4479BA; width: 300px;" | {{fontcolor|#FFF|Procedure}} | |||
! style="background: #4479BA; width: 300px;" | {{fontcolor|#FFF|Advantages}} | |||
! style="background: #4479BA; width: 300px;" | {{fontcolor|#FFF|Disadvantages}} | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| [[Thoracotomy]] (surgical opening of the chest) | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Allows the most thorough inspection and sampling of lymph node stations, may be followed by resection of tumor, if feasible | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Most invasive approach, not indicated for staging alone, significant risk of procedure-related morbidity | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Left parasternal mediastinotomy (or anterior mediastinotomy) | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Permits evaluation of the aortopulmonary window lymph nodes | |||
|style="padding: 5px 5px; background: #F5F5F5;"| More invasive; false-negative rate approximately 10%. | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Chamberlain procedure | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Access to station 5 ([[aortopulmonary window]] lymph node) | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Limited applications, invasive | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| [[Mediastinoscopy|Cervical mediastinoscopy]] | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Still considered the gold standard (usual comparitor) by many, excellent for 2RL 4RL | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Does not cover all medastinal lymph node stations, particularly subcarinal lymph nodes (station 7), paraesophageal and pulmonary ligament lymph nodes (stations 8 and 9), the aortopulmonary window lymph nodes (station 5), and the anterior mediastinal lymph nodes (station 6); false-negative rate approximately 20%; invasive | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| [[Thoracoscopy|Video-assisted thoracoscopy]] | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Good for inferior mediastinum, station 5 and 6 lymph nodes | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Invasive, does not cover superior anterior mediastinum | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Transthoracic percutaneous [[fine needle aspiration]] (FNA) under CT guidance | |||
|style="padding: 5px 5px; background: #F5F5F5;"| More widely available than some other methods | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Traverses a lot of lung tissue, therefore high pneumothorax risk, some lymph node stations inaccessible | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| [[Bronchoscopy]] with blind transbronchial FNA (Wang needle) | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Less invasive than above methods | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Relatively low yield, not widely practiced, bleeding risk | |||
|- | |||
|} | |||
{| style="border:1px solid black; border-collapse:collapse" border="1" cellpadding="5" | |||
|- | |||
! style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Procedure}} | |||
! style="background: #4479BA; width: 300px;" | {{fontcolor|#FFF|Advantages}} | |||
style="padding: 5px 5px; background: #F5F5F5;"| | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Endobronchial ultrasound (EBUS) | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Direct visualization of lymph node stations. | |||
*Complements EUS: covers lymph node stations 2R and 4R which are difficult to access by EUS | |||
*Lower false-negative rate than with blind transbronchial FNA and fewer complications | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*More invasive than EUS, few practitioners, but rapidly growing in popularity | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| [[Endoscopic ultrasound]] (EUS) | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Least invasive modality | |||
*Uses the esophagus to access mediastinal lymph nodes | |||
*Excellent for staging lymph nodes | |||
*Useful for station 2L and 4L, L adrenal, celiac lymph node | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Cannot reliably access right sided paratracheal lymph node stations 2 R and 4R | |||
*Accurate discrimination of primary hilar tumors and involved lymph nodes is important | |||
|} | |||
{| style="border: 0px; font-size: 90%; margin: 3px; width: 1000px" align=center | |||
|valign=top| | |||
|+ | |||
! style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Differential Diagnosis}} | |||
! style="background: #4479BA; width: 300px;" | {{fontcolor|#FFF|Similar Features}} | |||
! style="background: #4479BA; width: 300px;" | {{fontcolor|#FFF|Differentiating Features}} | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Pulmonary tuberculosis | |||
|style="padding: 5px 5px; background: #F5F5F5;"| Chronic [[cough]], [[weight loss]], [[hemoptysis]], nocturnal diaphoresis, [[dyspnea]] | |||
|style="padding: 5px 5px; background: #F5F5F5;"|In pulmonary tuberculosis, differentiating features include: increase in diameter despite optimal medical therapy, patients age is usually younger, hemoptisis is an early feature, and CXR anatomical predilection for upper lobes | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Lung abscess | |||
|style="padding: 5px 5px; background: #F5F5F5;"|Chronic [[cough]], [[weight loss]], [[hemoptysis]], and [[dyspnea]] | |||
|style="padding: 5px 5px; background: #F5F5F5;"|In lung abscess, differentiating features include: acute or subacute onset, CXR anatomical predilection for upper lobes, and usually resolve with antibiotic | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Pneumonia | |||
|style="padding: 5px 5px; background: #F5F5F5;"|Chronic [[cough]], [[weight loss]], [[hemoptysis]], and [[dyspnea]] | |||
|style="padding: 5px 5px; background: #F5F5F5;"|In pneumonia, differentiating features include: good response to antibiotics, acute onset, predilection on CXR is consolidation, laboratory markers indicate infection. | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Fungal infection | |||
|style="padding: 5px 5px; background: #F5F5F5;"|Chronic [[cough]], [[weight loss]], [[hemoptysis]], and [[dyspnea]] | |||
|style="padding: 5px 5px; background: #F5F5F5;"|In fungal infection, differentiating features include: CXR findings: air-cresecent sign, no response to antibioitcs, and mimcs tuberculosis. | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;"| Chronic eosinophilic pneumonia | |||
|style="padding: 5px 5px; background: #F5F5F5;"|Chronic [[cough]], [[weight loss]], [[hemoptysis]], and [[dyspnea]] | |||
|style="padding: 5px 5px; background: #F5F5F5;"|In chronic eosinophilic pneumonia , differentiating features include: followed by a parasite infection or medication exposure, and increased serum IgE levels | |||
|} | |||
{| style="border: 0px; font-size: 90%; margin: 3px; width: 1000px" align=center | |||
!style="padding: 5px 5px; background: #4479BA; font-weight: bold; text-align:center;" colspan="3"|{{fontcolor|#FFF|''Age-adjusted incidence of lung cancer by histological type'' <br><SMALL>Adapted from Wikipedia <ref name="wiki"> Lung Cancer Epidemiology. Wikipedia. https://en.wikipedia.org/wiki/Lung_cancer Accessed on February 17, 2016 </ref></SMALL>}} | |||
|valign=top| | |||
|+ | |||
|- | |||
| All types | |||
| 66.9 | |||
|- | |||
| Adenocarcinoma | |||
| 22.1 | |||
|- | |||
| Squamous-cell carcinoma | |||
| 14.4 | |||
|} | |||
{| style="border: 0px; font-size: 90%; margin: 3px; width: 1000px" align=center | |||
!style="padding: 5px 5px; background: #4479BA; font-weight: bold; text-align:center;" colspan="3"|{{fontcolor|#FFF|''Age-adjusted incidence of lung cancer by histological type'' <br><SMALL>Adapted from Wikipedia <ref name="wiki"> Lung Cancer Epidemiology. Wikipedia. https://en.wikipedia.org/wiki/Lung_cancer Accessed on February 17, 2016 </ref></SMALL>}} | |||
|valign=top| | |||
|+ | |||
!style="background: #4479BA; width: 50px;"|{{fontcolor|#FFF|'''Type'''}} | |||
!style="background: #4479BA; width: 200px;"|{{fontcolor|#FFF|''Incidence per 100,000 per year''}} | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC| All types | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
66.9 | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC| Adenocarcinoma | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
22.1 | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC| | |||
Squamous-cell carcinoma | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
14.4 | |||
|} | |||
{| style="border: 0px; font-size: 90%; margin: 3px; width: 1000px" align=center | |||
!style="padding: 5px 5px; background: #4479BA; font-weight: bold; text-align:center;" colspan="3"|{{fontcolor|#FFF|''Classification: Mucoepidermoid Carcinomas'' <br><SMALL>Adapted from Radiopedia <ref name="radiowiki"> Mucoepidermoid carcinoma. Radiopedia. Dr Frank Gailliard. http://radiopaedia.org/articles/mucoepidermoid-carcinoma-of-salivary-glands Accessed on February 17, 2016 </ref></SMALL>}} | |||
|valign=top| | |||
|+ | |||
|- | |||
|style="padding: 0 5px; background: #DCDCDC" rowspan="2"|{{fontcolor|#000|'''Salivary gland-confined carcinomas'''}} | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Major salivary glands (50%) | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Parotid gland (40%) | |||
*Submandibular gland (7%) | |||
*Sublingual gland (3%) | |||
|- | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Minor salivary glands (50%) | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Palate (most common) | |||
*Retromolar area | |||
*Floor of the mouth | |||
*Buccal mucosa | |||
*Lip | |||
*Tongue | |||
|- | |||
|style="padding: 0 5px; background: #DCDCDC" |{{fontcolor|#000|'''Other organ mucoepidermoid carcinomas'''}} | |||
|style="padding: 5px 5px; background: #F5F5F5;" colspan="2"| | |||
*Thyroid | |||
*Lung | |||
|} | |||
{|style="border: 0px; font-size: 90%; margin: 3px; width: 1000px" align=center | |||
!style="padding: 5px 5px; background: #4479BA; font-weight: bold; text-align:center;" colspan="3"|{{fontcolor|#FFF|''WHO histological classification system'' <br><SMALL>Adapted from WHO/IARC (2006) <ref name="radiowiki"> Tumors of the Lung. IARC/WHO https://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb10/bb10-chap1.pdf Accessed on February 22, 2016 </ref></SMALL>}} | |||
|valign=top| | |||
|+ | |||
|- | |||
| style="background: #4479BA; width: 300px;"|{{fontcolor|#FFF|''' Main types'''}} | |||
| style="background: #4479BA; width: 300px;"|{{fontcolor|#FFF|''' Subtypes'''}} | |||
| style="background: #4479BA; width: 300px;"|{{fontcolor|#FFF|''' Prevalence'''}} | |||
|- | |||
|style="padding: 0 5px; background: #DCDCDC"| Adenocarcinoma | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Adenocarcinoma, mixed | |||
*Acinar adenocarcinoma | |||
*Papillary adenocarcinoma | |||
*Bronchioloalveolar carcinoma | |||
*Nonmucinous | |||
*Mucinous | |||
*Mixed nonmucinous and mucinous or indeterminate | |||
*Solid adenocarcinoma with mucin production | |||
*Fetal adenocarcinoma | |||
*Mucinous (“colloid”) carcinoma | |||
*Mucinous cystadenocarcinoma | |||
*Signet ring adenocarcinoma | |||
*Clear cell adenocarcinoma | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*40% of lung cancers | |||
|- | |||
|style="padding: 0 5px; background: #DCDCDC"| Squamous cell carcinoma | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Papillary | |||
*Clear cell | |||
*Small cell | |||
*Basaloid | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*25% of lung cancers | |||
|- | |||
|style="padding: 0 5px; background: #DCDCDC"| Large cell carcinoma | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Large cell neuroendocrine carcinoma | |||
*Basaloid carcinoma | |||
*Lymphoepithelioma-like carcinoma | |||
*Clear cell carcinoma | |||
*Large cell carcinoma with rhabdoid phenotype | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*10% of lung cancer | |||
|- | |||
|style="background: #4479BA; width: 100px; text-align:center;" colspan="3"|{{fontcolor|#FFF|'''Less common types'''}} | |||
|- | |||
|style="padding: 0 5px; background: #DCDCDC" | Adenosquamous carcinoma | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*No subtypes | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Less than 5% | |||
|- | |||
|style="padding: 0 5px; background: #DCDCDC" | Sarcomatoid carcinoma | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Pleomorphic carcinoma | |||
*Spindle cell carcinoma | |||
*Giant cell carcinoma | |||
*Carcinosarcoma | |||
*Pulmonary blastoma | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Less than 5% | |||
|- | |||
|style="padding: 0 5px; background: #DCDCDC" | Carcinoid tumor | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Typical carcinoid | |||
*Atypical carcinoid | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Less than 5% | |||
|- | |||
|style="padding: 0 5px; background: #DCDCDC" | Salivary gland tumor | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Mucoepidermoid carcinoma | |||
*Adenoid cystic carcinoma | |||
*Epithelial-myoepithelial carcinoma | |||
|style="padding: 5px 5px; background: #F5F5F5;"| | |||
*Less than 5% | |||
|} | |||
{| style="border: 0px; font-size: 90%; margin: 3px; width: 1000px" align=center | {| style="border: 0px; font-size: 90%; margin: 3px; width: 1000px" align=center | ||
Latest revision as of 15:53, 27 April 2016
| Genetic lesions and potential therapeutic drugs Adapted from Chiaretti et al |
||
|---|---|---|
| Genes involved | Therapeutic compound | |
| TAL1 | HDAC inhibitors | |
| Notch1 | Y-secretase inhibitors | |
| ABL1 | ABL1 kinase inhibitors | |
| JAK2 | JAK2 inhibitors | |
| JAK1 | Tyrosine kinase inhibitors | |
| WHO histological classification Tumors of the appendix Adapted from WHO/IARC |
||
|---|---|---|
| Epithelial tumors | ||
| ||
| Non-epithelial tumors | ||
| ||
| Secondary tumors | ||
| ||
| Hyperplastic polyp | ||
CIN is classified in grades:
| Histology Grade | Description | Image |
|---|---|---|
| – | Normal cervical epithelium | 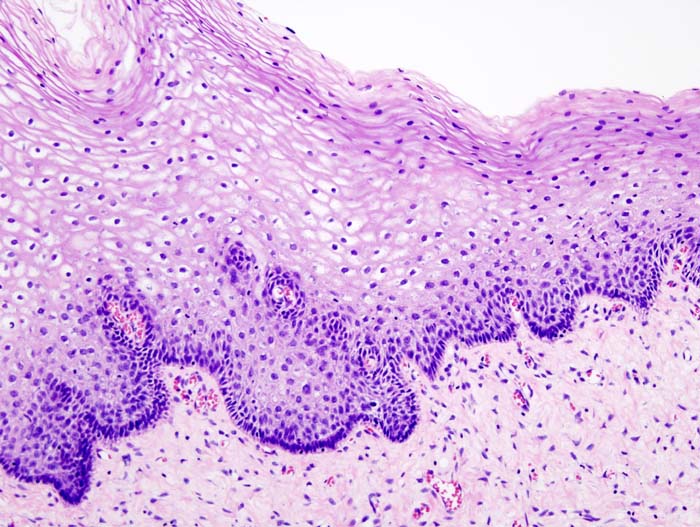 |
| CIN 1 (Grade I) | The least risky type, represents only mild dysplasia, or abnormal cell growth.It is confined to the basal 1/3 of the epithelium. This corresponds to infection with HPV, and typically will be cleared by immune response in a year or so, though can take several years to clear. | 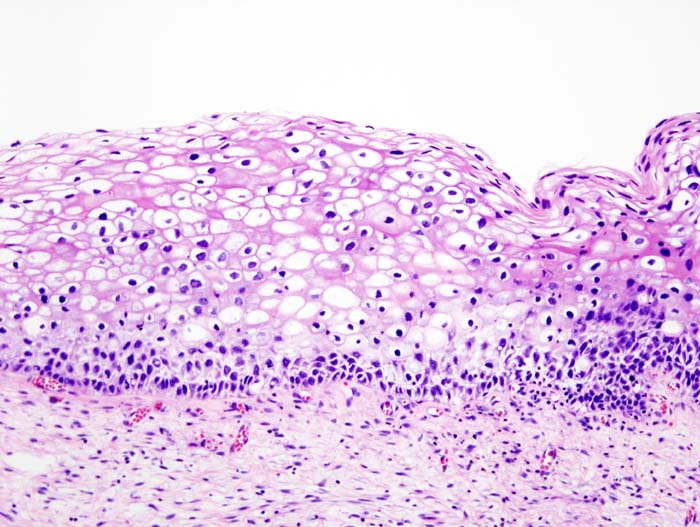 |
| CIN 2/3 | Formerly subdivided into CIN2 and CIN3. | |
| CIN 2 (Grade II) | Moderate dysplasia confined to the basal 2/3 of the epithelium | 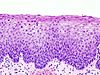 |
| CIN 3 (Grade III) | Severe dysplasia that spans more than 2/3 of the epithelium, and may involve the full thickness. This lesion may sometimes also be referred to as cervical carcinoma in situ. | 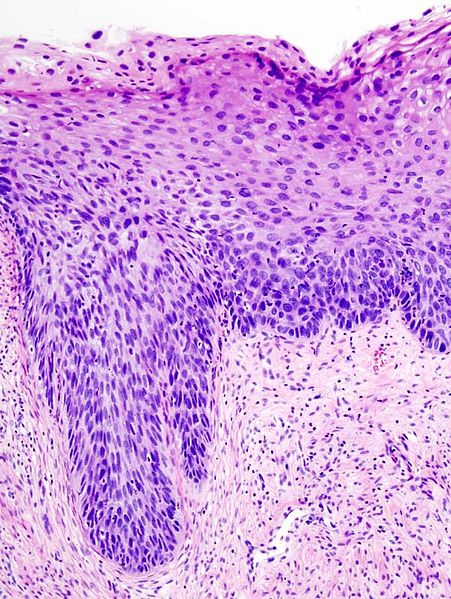 |
| Ultrasound findings of common liver masses | ||
|---|---|---|
| Common liver masses | Ultrasound finding | |
| Hepatic hemangioma |
| |
| Focal nodular hyperplasia |
| |
| Hepatic adenoma |
| |
| Idiopathic noncirrhotic portal hypertension |
| |
| Hepatocellular carcinoma |
| |
| Cholangiocarcinoma |
| |
| Metastases |
| |
| Classification of liver mass | ||
|---|---|---|
| Benign | Malignant | |
|
| |
| Cavitating causes | Conditions | Description |
|---|---|---|
| Malignancy |
Cancer
|
Cancer
|
| Infection |
|
Abscess:
Empyema:
|
|
Non-infectious |
|
|
|
Vascular |
|
|
|
Trauma |
|
|
| Congenital |
|
|
| Imaging features of lung mass | ||
|---|---|---|
| Hyperdense pulmonary mass | Cavitating pulmonary mass | |
|
Cancer
Autoimmune
Vascular
Infections (bacterial/fungal)
Trauma
Youth
| |
| Classification of Benign and Malignant Pulmonary Mass | |||
|---|---|---|---|
| Lung mass (location) | Benign | Malignant | |
| Endobronchial |
|
| |
| Parenchymal |
|
| |
| Pleural |
|
| |
| Radiologic Features Suggestive of Benign or Malignant Solitary Pulmonary Nodules Adapted from American Academy of Family Physicians [1] |
|||
|---|---|---|---|
| Radiologic feature | Benign | Malignant | |
| Size | < 5 mm | > 10 mm | |
| Border | Smooth | Irregular or spiculated | |
| Density | Dense, solid | Nonsolid, “ground glass” | |
| Calcification | Typically a benign feature, especially in “concentric,” “central,” “popcorn-like,” or “homogeneous” patterns | Typically noncalcified, or “eccentric” calcification | |
| Doubling time | Less than one month; more than one year | One month to one year | |
Recommendations for Follow-up and Management of Nodules <8 mm Detected Incidentally at Non-screening CT
| Nodule Size (mm) | Low risk patients | High risk patients |
|---|---|---|
| Less than or equal to 4 | |No follow-up needed. | Follow-up at 12 months. If no change, no further imaging needed. |
| >4 - 6 | Follow-up at 12 months. If no change, no further imaging needed. | Initial follow-up CT at 6 -12 months and then at 18 - 24 months if no change. |
| >6 - 8 | Initial follow-up CT at 6 -12 months and then at 18 - 24 months if no change. | Initial follow-up CT at 3 - 6 months and then at 9 -12 and 24 months if no change. |
| > 8 | Follow-up CTs at around 3, 9, and 24 months. Dynamic contrast enhanced CT, PET, and/or biopsy | Same at for low risk patients |
Note: Newly detected indeterminate nodule in persons 35 years of age or older.[2]
- Low risk patients: Minimal or absent history of smoking and of other known risk factors.
- High risk patients: History of smoking or of other known risk factors.
| Differential Diagnosis for Solitary Pulmonary Adapted from Erasmus et al. [1] |
||
|---|---|---|
| Differential Diagnosis | Causes | |
| Malignant neoplasms |
| |
| Benign neoplasms |
| |
| Infectious inflammatory |
| |
| Non-infectious inflammatory |
| |
| Vascular |
| |
| Congenital |
| |
| Miscellaneous |
| |
| Differential Diagnosis | Similar Features | Differentiating Features |
|---|---|---|
| Pulmonary tuberculosis |
|
|
| Lung abscess |
|
|
| Pneumonia |
|
|
| Pulmonary fungal infection |
|
|
| Other non-small cell lung cancers (adenocarcinoma and squamous cell lung cancer) |
|
|
| Differential Diagnosis | Similar Features | Differentiating Features |
|---|---|---|
| Pulmonary tuberculosis |
|
|
| Sarcoidosis |
|
|
| Pneumonia |
|
|
| Pulmonary fungal infection |
|
|
| Metastases |
|
|
| Stage (TNM criteria) | Standard Treatment Options |
|---|---|
| Occult |
|
| Stage 0 |
|
| Stages IA and IB |
|
| Stages IIA and IIB |
|
| Stage IIIA |
Resected or resectable disease
Unresectable disease
Superior sulcus tumors
Tumors that invade the chest wall
|
| Stage IIIB |
|
| Stage IV |
Maintenance therapy following first-line chemotherapy
|
| Recurrent |
|
| Type of tumor | Biopsy findings |
|---|---|
| Lung adenocarcinoma |
|
| Squamous cell lung carcinoma |
|
| Large cell lung carcinoma |
|
| Adenosquamous carcinoma |
|
| Sarcomatoid carcinoma |
|
| Carcinoid tumor |
|
| Salivary gland tumor |
|
| Organization Screening Guidelines for Non Small Cell Lung Cancer Adapted from CDC (2016) [1] |
|||
|---|---|---|---|
| Organization | Groups eligible for screening | Year | |
| American Academy of Family Practice | Evidence is insufficient to recommend for or against screening | 2013 | |
| American Association of Thoracic Surgery |
1. Age 55 to 79 years with 30 pack year smoking history. 2. Long term lung cancer survivors who have completed 4 years of surveillance without recurrence and who can tolerate lung cancer treatment following screening to detect second primary lung cancer until the age of 79. 3. Age 50 to 79 years with a 20 pack year smoking history and additional comorbidity that produces a cumulative risk of developing lung cancer ≥ 5% in 5 years |
2012 | |
| American Cancer Society |
Age 55 to 74 years with ≥30 pack year smoking history, who either currently smoke or have quit within the past 15 years, and who are in relatively good health. |
2015 | |
| American College of Chest Physicans |
Age 55 to 74 years with ≥30 pack year smoking history,who either currently smoke or have quit within the past 15 years |
2013 | |
| American Society of Clinical Oncology |
Age 55 to 74 years with ≥30 pack year smoking history,who either currently smoke or have quit within the past 15 years |
2012 | |
| American Lung Association |
Age 55 to 74 years with ≥ 30 pack year smoking history and no history of lung cancer |
2012 | |
| Medicaid Services |
Age 55 to 77 years with ≥ 30 pack year smoking history and smoking cessation < 15 years |
2015 | |
| National Comprehensive Cancer Network |
Age 55 to74 years with ≥30 packyear smoking history and smoking cessation < 15 years OR Age ≥ 50 years and ≥20 pack year smoking history and additional risk factor (other than secondhand smoke exposure |
2015 | |
| U.S Preventive Services Task Force |
Age 55 to 80 years with ≥30 pack year smoking history and smoking cessation < 15 years. |
2013 | |
| Procedure | Advantages | Disadvantages |
|---|---|---|
| Thoracotomy (surgical opening of the chest) | Allows the most thorough inspection and sampling of lymph node stations, may be followed by resection of tumor, if feasible | Most invasive approach, not indicated for staging alone, significant risk of procedure-related morbidity |
| Left parasternal mediastinotomy (or anterior mediastinotomy) | Permits evaluation of the aortopulmonary window lymph nodes | More invasive; false-negative rate approximately 10%. |
| Chamberlain procedure | Access to station 5 (aortopulmonary window lymph node) | Limited applications, invasive |
| Cervical mediastinoscopy | Still considered the gold standard (usual comparitor) by many, excellent for 2RL 4RL | Does not cover all medastinal lymph node stations, particularly subcarinal lymph nodes (station 7), paraesophageal and pulmonary ligament lymph nodes (stations 8 and 9), the aortopulmonary window lymph nodes (station 5), and the anterior mediastinal lymph nodes (station 6); false-negative rate approximately 20%; invasive |
| Video-assisted thoracoscopy | Good for inferior mediastinum, station 5 and 6 lymph nodes | Invasive, does not cover superior anterior mediastinum |
| Transthoracic percutaneous fine needle aspiration (FNA) under CT guidance | More widely available than some other methods | Traverses a lot of lung tissue, therefore high pneumothorax risk, some lymph node stations inaccessible |
| Bronchoscopy with blind transbronchial FNA (Wang needle) | Less invasive than above methods | Relatively low yield, not widely practiced, bleeding risk |
| Procedure | Advantages
style="padding: 5px 5px; background: #F5F5F5;"| | |
|---|---|---|
| Endobronchial ultrasound (EBUS) |
|
|
| Endoscopic ultrasound (EUS) |
|
|
| Differential Diagnosis | Similar Features | Differentiating Features |
|---|---|---|
| Pulmonary tuberculosis | Chronic cough, weight loss, hemoptysis, nocturnal diaphoresis, dyspnea | In pulmonary tuberculosis, differentiating features include: increase in diameter despite optimal medical therapy, patients age is usually younger, hemoptisis is an early feature, and CXR anatomical predilection for upper lobes |
| Lung abscess | Chronic cough, weight loss, hemoptysis, and dyspnea | In lung abscess, differentiating features include: acute or subacute onset, CXR anatomical predilection for upper lobes, and usually resolve with antibiotic |
| Pneumonia | Chronic cough, weight loss, hemoptysis, and dyspnea | In pneumonia, differentiating features include: good response to antibiotics, acute onset, predilection on CXR is consolidation, laboratory markers indicate infection. |
| Fungal infection | Chronic cough, weight loss, hemoptysis, and dyspnea | In fungal infection, differentiating features include: CXR findings: air-cresecent sign, no response to antibioitcs, and mimcs tuberculosis. |
| Chronic eosinophilic pneumonia | Chronic cough, weight loss, hemoptysis, and dyspnea | In chronic eosinophilic pneumonia , differentiating features include: followed by a parasite infection or medication exposure, and increased serum IgE levels |
| Age-adjusted incidence of lung cancer by histological type Adapted from Wikipedia [3] |
|||
|---|---|---|---|
| All types | 66.9 | ||
| Adenocarcinoma | 22.1 | ||
| Squamous-cell carcinoma | 14.4 | ||
| Age-adjusted incidence of lung cancer by histological type Adapted from Wikipedia [3] |
|||
|---|---|---|---|
| Type | Incidence per 100,000 per year | ||
| All types |
66.9 | ||
| Adenocarcinoma |
22.1 | ||
|
Squamous-cell carcinoma |
14.4 | ||
| Classification: Mucoepidermoid Carcinomas Adapted from Radiopedia [4] |
|||
|---|---|---|---|
| Salivary gland-confined carcinomas |
|
| |
|
| ||
| Other organ mucoepidermoid carcinomas |
| ||
| WHO histological classification system Adapted from WHO/IARC (2006) [4] |
|||
|---|---|---|---|
| Main types | Subtypes | Prevalence | |
| Adenocarcinoma |
|
| |
| Squamous cell carcinoma |
|
| |
| Large cell carcinoma |
|
| |
| Less common types | |||
| Adenosquamous carcinoma |
|
| |
| Sarcomatoid carcinoma |
|
| |
| Carcinoid tumor |
|
| |
| Salivary gland tumor |
|
| |
| Genes | Presence in non small cell-lung cancers |
|---|---|
| EGFR |
|
| KRAS |
|
| ALK |
|
| HER2 |
|
| BRAF |
|
| ROS-1 |
|
|
Classification | ||
|---|---|---|
| Salivary gland-confined carcinomas |
|
|
|
| |
| Other organ mucoepidermoid carcinomas |
| |
|
Mucoepidermoid carcinoma staging | |
|---|---|
| Tumor |
|
| |
| |
| |
| |
| Nodes |
|
| |
| |
| |
| |
| Overall stage |
|
| |
| |
| |
| |
|
| Differential Diagnosis | Similar Features | Differentiating Features |
|---|---|---|
| Benign mixed tumor | Painless parotid swelling and facial deformity | In benign mixed tumor , differentiating features include: histopathological findings |
| Warthin tumor | Painless swelling and facial deformity | In warthin tumor differentiating features include: multicentric presentation (20%) and are usually small (1-4 cm), highly associated with smoking |
| Adenoid cystic carcinoma | Swelling on salivary gland and facial deformity | In adenoid cystic carcinoma, differentiating features include: tendency for perineural extension, distribution, and mainly occur in relation to the airways |
| Metastasis | Painless swelling and facial deformity | In metastasis, differentiating features include: primary tumor origin, and histopathological findings. |
| Type of tumor | Age | Location | Histological features | Imaging features | Origin | Bone/Cartilage |
|---|---|---|---|---|---|---|
| Osteoma | 40-50 years | Skull bones | Matured lamellar bone | Sclerotic | Benign | Bone |
| Osteoid osteoma | 10-20 years | Short and long bone diaphysis | Osteiod outlined by osteoblasts, incorporated in a fibrous stroma | Sclerotic | Benign | Bone |
| Osteosarcoma | 11-40 years | Long bones metaphysis | Osteoid and bone formed of malignant osteoblasts and fibroblasts | Sclerotic | Malignant | Bone |
| Chondroma | 30-60 years | Small tubular bones of the hands and feet | Maturated hyaline cartilage (enchondroma/ecchondroma), preserving lobulation | Well-defined | Malignant | Cartilage |
| Chondrosarcoma | 30-60 years | Long bones metaphysic, axial skeleton | Immature cartilage, no preserving lobulation, cells arranged in groups of two or four, with atypia and mitosis | Well-defined | Malignant | Cartilage |
| Ewing sarcoma | 5-25 years | Long bones diaphysis | Small, round, undifferentiated cells, no stroma, a lot of capillary arrangement. | Ill-defined | Malignant | Bone |
| Giant cell tumor | 20-40 years | Knee | Multinucleated giant cells, fusiform cells, mononuclear cells. | Well-defined | Malignant | Bone |
| Metastases | 50-90 years | No site predilection | Frequently adenocarcinomas. Metastases can be blastic or lytic depending on the tumor origin | Sclerotic | Malignant | Bone |
| Stage | Description |
|---|---|
| I |
|
| II |
|
| III |
|
| Differential Diagnosis | Similar Features | Differentiating Features |
|---|---|---|
| Enchondroma |
|
|
| Chondroblastoma |
|
|
| Periosteal chondroma |
|
|
| Chondromyxoid fibroma |
|
|
| Type of osteochondroma | Features |
|---|---|
| Solitary osteochondroma |
|
| Multiple osteochondromas (hereditary) |
|
| Genes implicated in HNPCC | Frequency of mutations in HNPCC families | Locus |
|---|---|---|
| MSH2 | approximately 60% | 2p22 |
| MLH1 | approximately 30% | 3p21 |
| MSH6 | 7-10% | 2p16 |
| PMS2 | relatively infrequent | 7p22 |
| PMS1 | case report | 2q31-q33 |
| TGFBR2 | case report | 3p22 |
| MLH3 | disputed | 14q24.3 |
| Type of osteoid osteoma | Characteristics |
|---|---|
| Intracortical | Dense sclerosis around the nidus |
| Periosteal | Periosteal reaction |
| Cancellous (medullary) | Produces very little reactive bone |
| Subarticular | Simulates arthritis as it produces synovial reactions |
| Differential Diagnosis | Similar Features | Differentiating Features |
|---|---|---|
| Osteoblastoma |
|
|
| Brodie abscess |
|
|
| Osteosarcoma |
|
|
| Enostosis |
|
|
| Differential Diagnosis | Similar Features | Differentiating Features |
|---|---|---|
| Fibrous dysplasia |
|
|
| Osteoblastoma |
|
|
| Adamantinomas |
|
|
| Chronic sinusitis |
|
|
| Differential Diagnosis | Similar Features | Differentiating Features |
|---|---|---|
| Cardiac tamponade |
|
|
| Chronic obstructive pulmonary disease |
|
|
| Mediastinitis |
|
|
| Pneumonia |
|
|
| Acute respiratory distress syndrome |
|
|
| Syphilis |
|
|
| Differential Diagnosis | Similar Features | Differentiating Features |
|---|---|---|
| Familial adenomatous polyposis (FAP) |
|
|
| Juvenile polyposis |
|
|
| Cowden syndrome |
|
|
- ↑ 1.0 1.1 1.2 Solitary Pulmonary Nodule: Morphological Evaluation. http://pubs.rsna.org/doi/pdf/10.1148/radiographics.20.1.g00ja0343 Accessed on March 15, 2016
- ↑ Heber MacMahon, John H. M. Austin, Gordon Gamsu, Christian J. Herold, James R. Jett, David P. Naidich, Edward F. Patz, Jr, and Stephen J. Swensen. Guidelines for Management of Small Pulmonary Nodules Detected on CT Scans: A Statement from the Fleischner Society. Radiology 2005 237: 395-400.
- ↑ 3.0 3.1 Lung Cancer Epidemiology. Wikipedia. https://en.wikipedia.org/wiki/Lung_cancer Accessed on February 17, 2016
- ↑ 4.0 4.1 4.2 Mucoepidermoid carcinoma. Radiopedia. Dr Frank Gailliard. http://radiopaedia.org/articles/mucoepidermoid-carcinoma-of-salivary-glands Accessed on February 17, 2016
- ↑ AJCC System for Staging of Benign and Malignant Salivary Gland Tumors. AJCC Accessed on February 18, 2016