Sandbox:Dildar
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Dildar Hussain, MBBS [2]
PBC USG
There are no ultrasound findings associated with primary biliary cirrhosis. However, the ultrasound is mandatory for liver and biliary tree for all cholestatic patients for the differentiation of intrahepatic from extrahepatic cholestasis.
- The ultrasound findings may include:[1]
- Cholestasis
- Abdominal lymphadenopathy
Ultrasound examination of the liver and biliary tree is obligatory in all cholestatic patients in order to differentiate intrahepatic from extrahepatic . When the biliary system appears normal and serum AMA are present, no further radiologic workup is necessary. , particularly in the hilar region of the liver, is seen in 80% of patients with PBC
PBC CT
- Findings on CT scan suggestive of advanced primary biliary cirrhosis include:[2]
- Small heterogeneously attenuating liver
- Varices
- Splenomegaly
- Lymphadenopathy
- Findings on CT scan suggestive of less advanced disease include:
- Enlarged or normal size liver
- Smooth contour liver
- Little atrophy
- Lacelike fibrosis
- Regenerative nodules
- Varices
- Ascites
- Lymphadenopathy
Synonoms
- Solitary hyperplastic nodule
- Hepatic hamartoma
- Focal cirrhosis
- Hamartomatous cholangiohepatoma
- Hepatic pseudotumor
Historical Perspective
- In early 1900s,Focal nodular hyperplasia was first described.
- Between 1918-1982,96.625 autopsy studies were conducted out of which 8 percent of nonhemangiomatous lesions were focal nodular hyperplasia.
- In 1994,Working party of the world congresses of gastroenterology suggested a standardized terminology of nodular hepatic lesions that placed Focal noduldar carcinoma in the group of regenerative nodules, as opposed to dysplastic or neoplastic nodules.[3]
Differentiating Focal nodular hyperplasia from Other diseases
Focal nodular hyperplasia must be differentiated from:
- Hepatocellular carcinoma
- Cholangiocarcinoma
- Pancreatic carcinoma
- Liver hemangioma
- Liver abscess
- Cirrhosis
- Inflammatory lesions
Abbreviations: RUQ= Right upper quadrant of the abdomen, LUQ= Left upper quadrant, LLQ= Left lower quadrant, RLQ= Right lower quadrant, LFT= Liver function test, SIRS= Systemic inflammatory response syndrome, ERCP= Endoscopic retrograde cholangiopancreatography, IV= Intravenous, N= Normal, AMA= Anti mitochondrial antibodies, LDH= Lactate dehydrogenase, GI= Gastrointestinal, CXR= Chest X ray, IgA= Immunoglobulin A, IgG= Immunoglobulin G, IgM= Immunoglobulin M, CT= Computed tomography, PMN= Polymorphonuclear cells, ESR= Erythrocyte sedimentation rate, CRP= C-reactive protein, TS= Transferrin saturation, SF= Serum Ferritin, SMA= Superior mesenteric artery, SMV= Superior mesenteric vein, ECG= Electrocardiogram
| Disease | Clinical manifestations | Diagnosis | Comments | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptoms | Signs | |||||||||||||||
| Abdominal Pain | Fever | Rigors and chills | Nausea or vomiting | Jaundice | Constipation | Diarrhea | Weight loss | GI bleeding | Hypo-
tension |
Guarding | Rebound Tenderness | Bowel sounds | Lab Findings | Imaging | ||
| Focal nodular hyperplasia | Diffuse | ± | − | − | ± | − | − | + | + | − | − | − | Normal |
|
|
|
| Hepatocellular carcinoma/Metastasis | RUQ | + | − | + | + | + | + | + | + | + | − | + |
|
|
Other symptoms: | |
| Cholangiocarcinoma | RUQ | + | − | + | + | − | − | + | − | − | − | + | Normal |
|
| |
| Pancreatic carcinoma | MidEpigastric | − | − | + | + | + | − | + | − | − | − | + | Normal |
Skin manifestations may include: | ||
| Disease | Abdominal Pain | Fever | Rigors and chills | Nausea or vomiting | Jaundice | Constipation | Diarrhea | Weight loss | GI bleeding | Hypo-
tension |
Guarding | Rebound Tenderness | Bowel sounds | Lab Findings | Imaging | Comments |
| Gallbladder cancer | Midepigastric | − | − | + | + | − | + | + | − | − | − | − | Normal |
|
||
| Liver hemangioma | Intermittent RUQ | − | − | + | + | − | − | − | − | − | − | − | Normal |
|
| |
| Liver abscess | RUQ | + | − | + | + | − | − | + | − | − | − | − | Normal |
|
|
|
| Cirrhosis | RUQ+Bloating | + | − | + | + | − | − | + | − | − | − | − | Normal |
|
US
|
|
| Inflammatory lesions | RUQ | ± | − | + | + | − | − | − | − | − | − | − | Normal |
|
US
|
|
Focal Nodular Hyperplasia
FNH is typically benign, and usually no treatment is needed. Hemangiomas are the most common and are entirely benign. Treatment is unnecessary unless their expansion causes symptoms
Focal nodular hyperplasia (FNH) is a non-malignant hepatic tumor that is not of vascular origin. In one autopsy series of 96,625 patients, 8 percent of non-hemangiomatous lesions were FNH, representing 66 percent of all benign non-hemangiomatous lesions seen between 1918 and 1982 [1]. In large retrospective studies of patients referred for ultrasound and multidetector computed tomography, the prevalence of focal nodular hyperplasia was 0.2 percent and 1.6 percent, respectively [2,3].
FNH is seen in both sexes and throughout the age spectrum, although it is found predominantly in women (in a ratio of 8 or 9:1) between the ages of 20 and 50 years [4]. FNH comprises up to 2 percent of liver tumors in children [5].
This topic review will focus on the pathogenesis, clinical manifestations and management of FNH. An approach to patients presenting with a focal liver lesion is discussed separately. (See "Solid liver lesions: Differential diagnosis and evaluation".)
PATHOGENESIS — FNH has various labels: solitary hyperplastic nodule, hepatic hamartoma, focal cirrhosis, hamartomatous cholangiohepatoma, and hepatic pseudotumor. This profusion of terms epitomizes the confusion surrounding our understanding of the pathogenesis of the many conditions in which nodules of benign appearing hepatocytes are found. The International Working Party of the World Congresses of Gastroenterology proposed a standardized nomenclature in 1994, which placed FNH in the group of regenerative nodules, as opposed to dysplastic or neoplastic nodules [6]. This fits well with our current understanding of the pathogenesis of FNH. The contention that this lesion is non-neoplastic has been bolstered by the reported polyclonal origin of the hepatocytes [7], although this is disputed by others [8].
Previously considered to be a hamartoma, a neoplasm, a response to ischemia or other injury, or a focal area of regeneration, FNH is now generally accepted to be a hyperplastic (regenerative) response to hyperperfusion by the characteristic anomalous arteries found in the center of these nodules [4,9,10]. Whether vascular injury is also involved is less clear, but FNH is occasionally supplied primarily by portal venous blood due to thrombosis of the anomalous central artery [11].
The association of FNH with hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease [12]) and hepatic hemangiomas strengthens the hypothesis that FNH is a congenital vascular anomaly. Two pathology studies found cavernous hemangiomas in 6.5 and 2.3 percent of patients with FNH [13,14] and an imaging study, using ultrasound and dynamic CT, found that 23 percent of FNH patients had associated hemangiomas [15]. Multiple FNH lesions have also been noted in association with hemihypertrophy and vascular malformations (Klippel-Trénaunay-Weber syndrome) [16]. FNH with similar clinical and radiographic features has been documented in identical twins supporting a role of congenital vascular anomalies in its pathogenesis and a possible genetic predisposition to the disease [17].
PATHOLOGY — FNH is most often solitary (80 to 95 percent), and usually less than 5 cm in diameter. Only 3 percent are larger than 10 cm, although FNH as large as 19 cm have been reported [1,13,18]. It has a sharp margin with no capsule and may be pedunculated. The characteristic finding is the presence of a central stellate scar (picture 1) containing an inappropriately large artery with multiple branches radiating through the fibrous septa to the periphery. These branches divide the mass into multiple small nodules or cords of normal appearing hepatocytes (picture 2). The scar-like tissues within FNH nodules are composed of abnormally large portal tracts including large feeding arteries, portal veins, and bile ducts [10].
The arteries drain into adjacent hepatic veins. This radiating, branching pattern produces the spoke and wheel image typically seen on angiography. Although normal bile ducts are absent, bile ductules derived from hepatocyte metaplasia are usually prominent, traveling along the fibrous septa (picture 3). Sinusoids and Kupffer cells are typically present, distinguishing it from hepatocellular adenoma (HA), which usually lacks bile ducts and Kupffer cells [1,13,14,18,19]. The minimal microscopic criteria for the diagnosis of classical FNH are nodular architecture, abnormal vessels, and proliferation of bile ductules [13]. Lymphocyte infiltration, canalicular bile plugs, copper deposition, and feathery degeneration of hepatocytes may suggest cholestasis and/or inactive cirrhosis. Irregular intimal fibrosis or hypertrophy of the media may be seen in large arteries and veins, at times even occluding the lumen [13,14,19]. When present, portal veins are dilated and/or stenotic [10].
Non-classical variants — Non-classical forms of FNH lack either the typical nodular architecture or vascular malformations, but always contain bile ductular proliferation. They almost always lack the characteristic central scar [13]. Three variants have been recognized:
●The most common of these, the telangiectatic type, often presents with multiple FNH. In addition to the lack of a central scar, the mass is characterized by the absence of nodular architecture and the presence of single, quite regular plates of hepatocytes separated by sinusoids fed directly by anomalous arteries [13,20]. The risk of bleeding appears to be similar to the risk observed in patients with a hepatic adenoma [21].
●A mixed hyperplastic and adenomatous form may be difficult to distinguish from HA due to its subtle vascular and bile ductular findings [13,20].
●A third histologic variant consisting of FNH with cytologic atypia resembling dysplasia of large cell type has been proposed [13].
A comprehensive pathological study of 305 lesions failed to identify a macroscopic central stellate scar in 50 percent and noted non-classical histology in 20 percent of the lesions, most showing a telangiectatic variant [13]. The surprisingly high number of lesions without a central scar was almost exclusively due to the large number of masses that had non-classical histology. Ninety-five percent of those with non-classical histology did not have a scar, whereas only 18 percent of those with classical histology lacked a scar [13]. The overall prevalence and clinical significance of these variants remains to be determined.
DIAGNOSIS — The diagnosis of FNH is usually made by demonstrating its characteristic features on imaging tests and excluding other lesions. The latter can typically be accomplished by assessment of the context in which FNH is detected and by obtaining specific radiologic and laboratory testing (table 1). The differential diagnosis includes hepatic adenoma, hepatocellular carcinoma, fibrolamellar carcinoma, cirrhosis, large regenerative nodules, hemangioma, and hypervascular metastases. (See "Solid liver lesions: Differential diagnosis and evaluation".)
Symptoms — The majority of reports have found that symptoms or signs directly attributable to FNH are infrequent. Two-thirds to three-fourths of patients are identified incidentally [18], with the mass noted at the time of surgery, on an abdominal imaging study, or at autopsy. Unlike hepatic adenomas, FNH rarely presents with acute onset of hemorrhage, necrosis, or infarction [22,23].
However, symptomatic presentations have been described. In one series, for example, abdominal discomfort or a palpable liver mass was observed in 25 percent of 41 patients [24]. Another series that included 168 patients found that 60 percent had abdominal pain and 4 percent had an abdominal mass [13]. The high number of symptomatic patients in the second report probably reflects selection bias since all of the patients were identified from pathology specimens obtained at the time of surgical resection [13].
Laboratory tests — Liver tests are most often normal although minor elevations in aspartate and alanine aminotransferase, alkaline phosphatase and gamma glutamyl transpeptidase levels may be seen [13,14,24]. The alpha-fetoprotein is normal.
Imaging tests — A confident diagnosis can usually be made through a combination of imaging modalities; tissue diagnosis is usually not required.
Ultrasonography — Although often first identified on ultrasound examination, FNH is variably hyper, hypo, or isoechoic [24] and US is able to identify the central scar in only 20 percent of cases [25]. The ultrasound characteristics are difficult to distinguish from an adenoma or malignant lesions. Power Doppler ultrasound may help differentiate the arterial flow in FNH from the venous flow in HA [24,26,27].
Contrast-enhanced ultrasonography — Several reports have described improved characterization of focal liver lesions using contrast-enhanced ultrasonography compared with standard ultrasonography [28-30]. While the approach is not approved in the United States, it is available in other countries. Test characteristics compared with other imaging modalities remain incompletely defined, although emerging data suggest its ability for differentiation among solid liver lesions is comparable with MRI [31]. (See "Contrast-enhanced ultrasound for the evaluation of liver lesions".)
CT scan — A properly timed dynamic, triphasic, helical CT scan performed without contrast, and with contrast during the hepatic arterial and portal venous phases, will often be highly suggestive of the diagnosis [32,33]. The lesion may be hypo or isodense on non-contrast imaging with the central scar identified in one-third of patients. The lesion becomes hyperdense during the hepatic arterial phase due to the arterial origin of its blood supply (image 1). FNH is generally isodense during the portal venous phase, although the central scar may become hyperdense as contrast diffuses into the scar. While characteristic of FNH, a central scar may be present in the fibrolamellar variant of HCC. (See "Epidemiology, clinical manifestations, diagnosis, and treatment of fibrolamellar carcinoma", section on 'Imaging'.)
MRI — There may be little to distinguish FNH from normal liver on standard MRI, since it is composed of the same elements as normal liver. An isointense lesion is noted on T1-weighted images, while an isointense to slightly hyperintense mass appears on T2-weighted images (image 2 and image 3) [34]. The scar typically shows high signal intensity on T2-weighted images due to vessels or edema in the scar (image 3) [35]. Gadolinium infusion produces rapid enhancement of the FNH mass due to its arterial blood supply, producing a hyperintense lesion on early films (image 2). On delayed images it becomes more isointense with respect to normal liver. The central scar enhances on delayed imaging as contrast gradually diffuses into the fibrous center of the mass [36-39]. In one study, gadolinium enhanced MRI had a sensitivity and specificity of 70 and 98 percent, respectively [24].
A relatively new MR contrast agent has been introduced into clinical use. Unlike currently used gadolinium-based contrast agents for MRI, this agent, a Gd-BOPTA chelate of Gadobenate Dimeglumine, has a dual route of elimination, through both renal and hepatobiliary excretion (image 4). Thus, it can be useful for distinguishing hepatic adenomas from focal nodular hyperplasia. (See "Solid liver lesions: Differential diagnosis and evaluation".)
Angiography — Although angiography may reveal the diagnostic "spoked wheel" appearance of FNH, its use is rarely indicated [32,40,41].
ROLE OF ORAL CONTRACEPTIVES — FNH was first described in the early 1900s, long before the advent of oral contraceptives (OCPs). It is seen in men and children who do not use OCPs and its incidence remained steady after the introduction of OCPs in 1960, in sharp contrast to the dramatic rise in the incidence of HA with the widespread use of OCPs. Thus use of OCPs is not required for the development of FNH [42-44].
On the other hand, FNH may be responsive to estrogens [11]. Patients taking OCPs tend to have larger, more vascular tumors, have more symptoms, and reports of hemorrhage or rupture in patients with FNH have all occurred in patients taking OCPs [45-48]. However, the magnitude of the risk associated with OCPs is uncertain. In a study of 216 women with FNH, use of OCPs did not appear to influence the size or number of FNH lesions or size changes (which were rare) during follow-up for an average of two years [49]. A case control trial comparing 23 women with histologically confirmed FNH to 94 controls estimated the odds ratio of OCP use to be 2.8 (95% CI, 0.8 to 9.4) for those who had ever used OCPs and 4.5 (95% CI, 1.2 to 16.9) for those who had ≥3 years of use [50].
We generally do not insist that oral contraceptives and other estrogen containing preparations should be discontinued. However, it is reasonable to obtain a follow-up imaging study in 6 to 12 months in women who continue taking these drugs.
MANAGEMENT — The natural history of FNH is one of stability and lack of complications. Lesions generally do not change over time, although they occasionally become smaller [49,51-54]. However, as mentioned above, enlargement of FNH in the setting of OCPs and during pregnancy have been reported [55]. There is no evidence for malignant transformation of FNH [13,24,56,57].
Patients who are suspected of having FNH based upon the evaluation described above should be managed conservatively [24,35,49,51,52,54,58,59]. If a diagnosis remains unclear, a liver biopsy may be helpful, but may also be misleading since only resection will be definitive [60]. Follow-up studies at three and six months will often be sufficient to confirm the stability of the lesion and its benign nature, after which no long-term follow-up is required routinely. Surgery should be reserved for the rare, very symptomatic FNH lesion, and the highly suspicious lesion, which has eluded diagnosis by all other modalities.
We generally do not insist that oral contraceptives and other estrogen containing preparations should be discontinued. However, it is reasonable to obtain a follow-up imaging study in 6 to 12 months in women who continue taking these drugs. Small FNH do not appear to pose a significant risk to a successful pregnancy [49,61], although close observation is strongly recommended and resection may be prudent for large (>8 cm) FNH.
SUMMARY AND RECOMMENDATIONS
●Focal nodular hyperplasia (FNH) is a non-malignant hepatic tumor that is not of vascular origin. It is now generally accepted to be a hyperplastic (regenerative) response to hyperperfusion by the characteristic anomalous arteries found in the center of these nodules. (See 'Pathogenesis' above.)
●FNH is most often solitary (80 to 95 percent) and usually less than 5 cm in diameter. Only 3 percent are larger than 10 cm. (See 'Pathology' above.)
●The majority of reports have found that symptoms or signs directly attributable to FNH are infrequent. (See 'Symptoms' above.)
●The diagnosis of FNH is usually made by demonstrating its characteristic features on imaging tests and excluding other lesions. The latter can typically be accomplished by assessing the context in which FNH is detected and by obtaining specific radiologic and laboratory testing (table 1). (See 'Diagnosis' above and "Solid liver lesions: Differential diagnosis and evaluation".)
●The natural history of FNH is one of stability and a lack of complications. Thus, we suggest that patients who are suspected of having FNH based upon the evaluation described above be managed conservatively (Grade 2B). (See 'Management' above.)
●FNH may be responsive to exogenous estrogens. We generally do not insist that oral contraceptives and other estrogen-containing preparations should be discontinued. However, it is reasonable to obtain a follow-up imaging study in 6 to 12 months in women who continue taking these drugs. (See 'Role of oral contraceptives' above.)
Use of UpToDate is subject to the Subscription and License Agreement.
REFERENCES
1.Craig J, Peters R, Edmundson H. Tumors of the Liver and Intrahepatic Bile Ducts, Fasc 26, 2nd ed, DC Armed Forces Institute of Pathology, Washington, DC 1989. p.6.
2.Kaltenbach TE, Engler P, Kratzer W, et al. Prevalence of benign focal liver lesions: ultrasound investigation of 45,319 hospital patients. Abdom Radiol (NY) 2016; 41:25.
3.Horta G, López M, Dotte A, et al. [Benign focal liver lesions detected by computed tomography: Review of 1,184 examinations]. Rev Med Chil 2015; 143:197.
4.Wanless IR, Mawdsley C, Adams R. On the pathogenesis of focal nodular hyperplasia of the liver. Hepatology 1985; 5:1194.
5.Reymond D, Plaschkes J, Lüthy AR, et al. Focal nodular hyperplasia of the liver in children: review of follow-up and outcome. J Pediatr Surg 1995; 30:1590.
6.International Working Party. Terminology of nodular hepatocellular lesions. Hepatology 1995; 22:983.
7.Paradis V, Laurent A, Flejou JF, et al. Evidence for the polyclonal nature of focal nodular hyperplasia of the liver by the study of X-chromosome inactivation. Hepatology 1997; 26:891.
8.Gaffey MJ, Iezzoni JC, Weiss LM. Clonal analysis of focal nodular hyperplasia of the liver. Am J Pathol 1996; 148:1089.
9.Fukukura Y, Nakashima O, Kusaba A, et al. Angioarchitecture and blood circulation in focal nodular hyperplasia of the liver. J Hepatol 1998; 29:470.
10.Kondo F, Nagao T, Sato T, et al. Etiological analysis of focal nodular hyperplasia of the liver, with emphasis on similar abnormal vasculatures to nodular regenerative hyperplasia and idiopathic portal hypertension. Pathol Res Pract 1998; 194:487.
11.Nakanuma Y. Non-neoplastic nodular lesions in the liver. Pathol Int 1995; 45:703.
12.Wanless IR, Gryfe A. Nodular transformation of the liver in hereditary hemorrhagic telangiectasia. Arch Pathol Lab Med 1986; 110:331.
13.Nguyen BN, Fléjou JF, Terris B, et al. Focal nodular hyperplasia of the liver: a comprehensive pathologic study of 305 lesions and recognition of new histologic forms. Am J Surg Pathol 1999; 23:1441.
14.Ishak KG, Rabin L. Benign tumors of the liver. Med Clin North Am 1975; 59:995.
15.Mathieu D, Zafrani ES, Anglade MC, Dhumeaux D. Association of focal nodular hyperplasia and hepatic hemangioma. Gastroenterology 1989; 97:154.
16.Haber M, Reuben A, Burrell M, et al. Multiple focal nodular hyperplasia of the liver associated with hemihypertrophy and vascular malformations. Gastroenterology 1995; 108:1256.
17.Mindikoglu AL, Regev A, Levi JU, et al. Focal nodular hyperplasia in identical twins. Am J Gastroenterol 2005; 100:1616.
18.Goodman ZD. Benign Tumors of the Liver. In: Neoplasms of the Liver, Okuda K, Ishak KD (Eds), Springer, Tokyo 1987. p.105.
19.Klatskin G, Conn H. Neoplasms of the Liver and Intrahepatic Bile Ducts. In: Histopathology of the Liver, Oxford University Press, New York 1993. p.367.
20.Wanless IR, Albrecht S, Bilbao J, et al. Multiple focal nodular hyperplasia of the liver associated with vascular malformations of various organs and neoplasia of the brain: a new syndrome. Mod Pathol 1989; 2:456.
21.Bioulac-Sage P, Rebouissou S, Sa Cunha A, et al. Clinical, morphologic, and molecular features defining so-called telangiectatic focal nodular hyperplasias of the liver. Gastroenterology 2005; 128:1211.
22.Brunt EM, Flye MW. Infarction in focal nodular hyperplasia of the liver. A case report. Am J Clin Pathol 1991; 95:503.
23.Lee MJ, Saini S, Hamm B, et al. Focal nodular hyperplasia of the liver: MR findings in 35 proved cases. AJR Am J Roentgenol 1991; 156:317.
24.Cherqui D, Rahmouni A, Charlotte F, et al. Management of focal nodular hyperplasia and hepatocellular adenoma in young women: a series of 41 patients with clinical, radiological, and pathological correlations. Hepatology 1995; 22:1674.
25.Shamsi K, De Schepper A, Degryse H, Deckers F. Focal nodular hyperplasia of the liver: radiologic findings. Abdom Imaging 1993; 18:32.
26.Bartolozzi C, Lencioni R, Paolicchi A, et al. Differentiation of hepatocellular adenoma and focal nodular hyperplasia of the liver: comparison of power Doppler imaging and conventional color Doppler sonography. Eur Radiol 1997; 7:1410.
27.Wang LY, Wang JH, Lin ZY, et al. Hepatic focal nodular hyperplasia: findings on color Doppler ultrasound. Abdom Imaging 1997; 22:178.
28.Quaia E. The real capabilities of contrast-enhanced ultrasound in the characterization of solid focal liver lesions. Eur Radiol 2011; 21:457.
29.Kang HS, Kim BK, Shim CS. Focal nodular hyperplasia: with a focus on contrast enhanced ultrasound. Korean J Hepatol 2010; 16:414.
30.Bernatik T, Seitz K, Blank W, et al. Unclear focal liver lesions in contrast-enhanced ultrasonography--lessons to be learned from the DEGUM multicenter study for the characterization of liver tumors. Ultraschall Med 2010; 31:577.
31.Seitz K, Bernatik T, Strobel D, et al. Contrast-enhanced ultrasound (CEUS) for the characterization of focal liver lesions in clinical practice (DEGUM Multicenter Trial): CEUS vs. MRI--a prospective comparison in 269 patients. Ultraschall Med 2010; 31:492.
32.Mergo PJ, Ros PR. Benign Lesions of the Liver. In: The Radiologic Clinics of North America, 2, W.B. Saunders, Philadelphia 1998. Vol 36, p.319.
33.Carlson SK, Johnson CD, Bender CE, Welch TJ. CT of focal nodular hyperplasia of the liver. AJR Am J Roentgenol 2000; 174:705.
34.Mattison GR, Glazer GM, Quint LE, et al. MR imaging of hepatic focal nodular hyperplasia: characterization and distinction from primary malignant hepatic tumors. AJR Am J Roentgenol 1987; 148:711.
35.Buetow PC, Pantongrag-Brown L, Buck JL, et al. Focal nodular hyperplasia of the liver: radiologic-pathologic correlation. Radiographics 1996; 16:369.
36.Irie H, Honda H, Kaneko K, et al. MR imaging of focal nodular hyperplasia of the liver: value of contrast-enhanced dynamic study. Radiat Med 1997; 15:29.
37.Mahfouz AE, Hamm B, Taupitz M, Wolf KJ. Hypervascular liver lesions: differentiation of focal nodular hyperplasia from malignant tumors with dynamic gadolinium-enhanced MR imaging. Radiology 1993; 186:133.
38.Rummeny E, Weissleder R, Sironi S, et al. Central scars in primary liver tumors: MR features, specificity, and pathologic correlation. Radiology 1989; 171:323.
39.Mathieu D, Rahmouni A, Anglade MC, et al. Focal nodular hyperplasia of the liver: assessment with contrast-enhanced TurboFLASH MR imaging. Radiology 1991; 180:25.
40.Welch TJ, Sheedy PF 2nd, Johnson CM, et al. Focal nodular hyperplasia and hepatic adenoma: comparison of angiography, CT, US, and scintigraphy. Radiology 1985; 156:593.
41.Rogers JV, Mack LA, Freeny PC, et al. Hepatic focal nodular hyperplasia: angiography, CT, sonography, and scintigraphy. AJR Am J Roentgenol 1981; 137:983.
42.Fechner RE. Benign hepatic lesions and orally administered contraceptives. A report of seven cases and a critical analysis of the literature. Hum Pathol 1977; 8:255.
43.Ishak KG. Hepatic Neoplasms Associated with Contraceptive and Anabolic Steroids in Carcinogenic Hormones. In: Recent Results in Cancer Research, Lingeman CH (Ed), Springer-Verlag, New York 1979. p.73.
44.Geders JM, Haque S, Tesi RJ, et al. A young man with a solitary hepatic mass. Hepatology 1995; 22:655.
45.Nime F, Pickren JW, Vana J, et al. The histology of liver tumors in oral contraceptive users observed during a national survey by the American College of Surgeons Commission on Cancer. Cancer 1979; 44:1481.
46.Aldinger K, Ben-Menachem Y, Whalen G. Focal nodular hyperplasia of the liver associated with high-dosage estrogens. Arch Intern Med 1977; 137:357.
47.Klatskin G. Hepatic tumors: possible relationship to use of oral contraceptives. Gastroenterology 1977; 73:386.
48.Shortell CK, Schwartz SI. Hepatic adenoma and focal nodular hyperplasia. Surg Gynecol Obstet 1991; 173:426.
49.Mathieu D, Kobeiter H, Maison P, et al. Oral contraceptive use and focal nodular hyperplasia of the liver. Gastroenterology 2000; 118:560.
50.Scalori A, Tavani A, Gallus S, et al. Oral contraceptives and the risk of focal nodular hyperplasia of the liver: a case-control study. Am J Obstet Gynecol 2002; 186:195.
51.Weimann A, Ringe B, Klempnauer J, et al. Benign liver tumors: differential diagnosis and indications for surgery. World J Surg 1997; 21:983.
52.Heinemann LA, Weimann A, Gerken G, et al. Modern oral contraceptive use and benign liver tumors: the German Benign Liver Tumor Case-Control Study. Eur J Contracept Reprod Health Care 1998; 3:194.
53.Di Stasi M, Caturelli E, De Sio I, et al. Natural history of focal nodular hyperplasia of the liver: an ultrasound study. J Clin Ultrasound 1996; 24:345.
54.Leconte I, Van Beers BE, Lacrosse M, et al. Focal nodular hyperplasia: natural course observed with CT and MRI. J Comput Assist Tomogr 2000; 24:61.
55.Scott LD, Katz AR, Duke JH, et al. Oral contraceptives, pregnancy, and focal nodular hyperplasia of the liver. JAMA 1984; 251:1461.
56.Wanless IR. Nodular regenerative hyperplasia, dysplasia, and hepatocellular carcinoma. Am J Gastroenterol 1996; 91:836.
57.Rubin RA, Mitchell DG. Evaluation of the solid hepatic mass. Med Clin North Am 1996; 80:907.
58.Belghiti J, Pateron D, Panis Y, et al. Resection of presumed benign liver tumours. Br J Surg 1993; 80:380.
59.De Carlis L, Pirotta V, Rondinara GF, et al. Hepatic adenoma and focal nodular hyperplasia: diagnosis and criteria for treatment. Liver Transpl Surg 1997; 3:160.
60.Fabre A, Audet P, Vilgrain V, et al. Histologic scoring of liver biopsy in focal nodular hyperplasia with atypical presentation. Hepatology 2002; 35:414.
61.Weimann A, Mössinger M, Fronhoff K, et al. Pregnancy in women with observed focal nodular hyperplasia of the liver. Lancet 1998; 351:1251.
HCC Differnetial Table
Abbreviations: RUQ= Right upper quadrant of the abdomen, LUQ= Left upper quadrant, LLQ= Left lower quadrant, RLQ= Right lower quadrant, LFT= Liver function test, SIRS= Systemic inflammatory response syndrome, ERCP= Endoscopic retrograde cholangiopancreatography, IV= Intravenous, N= Normal, AMA= Anti mitochondrial antibodies, LDH= Lactate dehydrogenase, GI= Gastrointestinal, CXR= Chest X ray, IgA= Immunoglobulin A, IgG= Immunoglobulin G, IgM= Immunoglobulin M, CT= Computed tomography, PMN= Polymorphonuclear cells, ESR= Erythrocyte sedimentation rate, CRP= C-reactive protein, TS= Transferrin saturation, SF= Serum Ferritin, SMA= Superior mesenteric artery, SMV= Superior mesenteric vein, ECG= Electrocardiogram
| Disease | Clinical manifestations | Diagnosis | Comments | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptoms | Signs | |||||||||||||||
| Abdominal Pain | Fever | Rigors and chills | Nausea or vomiting | Jaundice | Constipation | Diarrhea | Weight loss | GI bleeding | Hypo-
tension |
Guarding | Rebound Tenderness | Bowel sounds | Lab Findings | Imaging | ||
| Hepatocellular carcinoma/Metastasis | RUQ | + | − | + | + | + | + | + | + | + | − | + |
|
|
Other symptoms: | |
| Cholangiocarcinoma | RUQ | + | − | + | + | − | − | + | − | − | − | + | Normal |
|
| |
| Pancreatic carcinoma | MidEpigastric | − | − | + | + | + | − | + | − | − | − | + | Normal |
Skin manifestations may include: | ||
| Focal nodular hyperplasia | Diffuse | ± | − | − | ± | − | − | + | + | − | − | − | Normal |
|
|
|
| Disease | Abdominal Pain | Fever | Rigors and chills | Nausea or vomiting | Jaundice | Constipation | Diarrhea | Weight loss | GI bleeding | Hypo-
tension |
Guarding | Rebound Tenderness | Bowel sounds | Lab Findings | Imaging | Comments |
| Gallbladder cancer | Midepigastric | − | − | + | + | − | + | + | − | − | − | − | Normal |
|
||
| Liver hemangioma | Intermittent RUQ | − | − | + | + | − | − | − | − | − | − | − | Normal |
|
| |
| Liver abscess | RUQ | + | − | + | + | − | − | + | − | − | − | − | Normal |
|
|
|
| Cirrhosis | RUQ+Bloating | + | − | + | + | − | − | + | − | − | − | − | Normal |
|
US
|
|
| Inflammatory lesions | RUQ | ± | − | + | + | − | − | − | − | − | − | − | Normal |
|
US
|
|
Classification
Historical Perspective
Pathophysiology
Causes
Differentiating Splenic Rupture from Other Diseases
Epidemiology and Demographics
Risk Factors
Screening
Natural History, Complications and Prognosis
Diagnosis
Diagnostic Study of Choice
History and Symptoms
Physical Examination
Laboratory Findings
Electrocardiogram
X-Ray
MRI
Other Imaging Findings
Other Diagnostic Studies
Algorithms
| Major molecular events in the pathogenesis of HCC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Genomic alterations | Epigenetic modifications | Growthfactor pathway alterations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Mutations | Gene Amplification | DNA methylation micro RNA | Micro RNA | LNC RNA | Major Signaling pathways | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| •TERT promoter •TP53 •CTNNB1 •AXIN1 •AXIN2 •ATM •RPS6KA3 •JAK1 •IL6R •IL6ST •ARID1 •ARID2 | •CCND1 •FGF19 •CDKNA2A •CDKNA2B •AXIN1 •IRF2 •MET | GSTP1 •E-Cadherin •CDKNA2 •RASSF1A •SOCS-3 •MIGMT | •MiR-155 •Mir-122 •Mir-224 •Mir-21 | •HULC •HEIH •Dreh •MVIH •HOTAIR •MDIG •LINE1 | •Wnt/β –catenin •Tyrosine kinase pathways EGF HGF/c-MET FGF VEGF •IGF •HIF •TGF β •Hedgehog | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

The incidence of HCC has almost tripled since the early 1980s in the United States where it is the fastest rising cause of cancer-related deaths1. According to population based Surveillance Epidemiology and End Results registry data, the overall HCC age adjusted incidence rates for liver and intrahepatic ducts cancer is as high as 8 per 100,000 underling population in 2010 (Fig. 1) of which at least 6 per 100,000 related to HCC. Men are at approximately three times higher risk than women. Asian men (i.e., Chinese, Korean, Filipino, and Japanese) have the highest age-adjusted incidence rates. However, the largest proportional increases have occurred among Hispanics followed by blacks and non-Hispanic whites, whereas the lowest proportional increases have occurred among Asians. In contrast to Asians/Pacific Islanders, HCC incidence rates are reported to be higher among Hispanics born in the United States than among foreign-born Hispanics2. HCC incidence rates have increased in each successive birth cohort born between 1900 and 19593 (Fig. 2). In addition, the age distribution of HCC patients has shifted to younger ages, with the greatest proportional increases among individuals 45–60 years old (Fig. 2). There is a south to north gradient in the incidence and mortality of HCC; Southern states including Texas, Louisiana, and Mississippi have some of the highest HCC incidence rates in the nation (Fig. 3). In one study, Texas Latino and especially South Texas Latinos had the highest age-adjusted HCC incidence rates (as high as 10.6/100,000)4.
Video codes
Normal video
{{#ev:youtube|dU26cGlmkRg}} {{#ev:youtube|4uSSvD1BAHg}} {{#ev:youtube|PQXb5D-5UZw}} {{#ev:youtube|UVJYQlUm2A8}}
Video in table
Floating video
| Title |
| https://https://www.youtube.com/watch?v=o1VTHP_oYD8}} |
Redirect
- REDIRECTEsophageal web
synonym website
https://mq.b2i.sg/snow-owl/#!terminology/snomed/10743008
Image
Image to the right
 |
Image and text to the right
<figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline>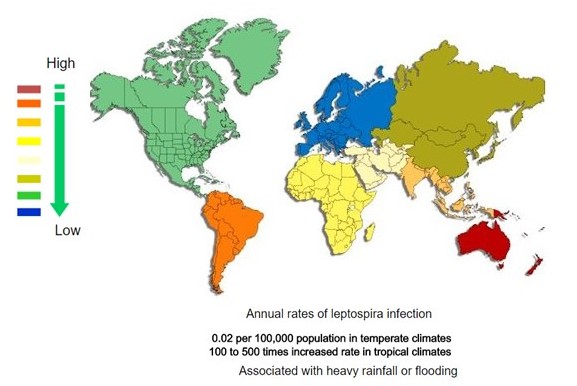 </figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline> Recent out break of leptospirosis is reported in Bronx, New York and found 3 cases in the months January and February, 2017.
</figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline> Recent out break of leptospirosis is reported in Bronx, New York and found 3 cases in the months January and February, 2017.
Gallery
-
Histopathology of a pancreatic endocrine tumor (insulinoma). Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[5]
-
Histopathology of a pancreatic endocrine tumor (insulinoma). Chromogranin A immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[5]
-
Histopathology of a pancreatic endocrine tumor (insulinoma). Insulin immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[5]
References
- ↑ Blachar A, Federle MP, Brancatelli G (2001). "Primary biliary cirrhosis: clinical, pathologic, and helical CT findings in 53 patients". Radiology. 220 (2): 329–36. doi:10.1148/radiology.220.2.r01au36329. PMID 11477233.
- ↑ Blachar, Arye; Federle, Michael P.; Brancatelli, Giuseppe (2001). "Primary Biliary Cirrhosis: Clinical, Pathologic, and Helical CT Findings in 53 Patients". Radiology. 220 (2): 329–336. doi:10.1148/radiology.220.2.r01au36329. ISSN 0033-8419.
- ↑ "Terminology of nodular hepatocellular lesions". Hepatology. 22 (3): 983–93. 1995. PMID 7657307.
- ↑ "File:Jaundice08.jpg - Wikimedia Commons". External link in
|title=(help) - ↑ 5.0 5.1 5.2 Neuroendocrine tumor of the pancreas. Libre Pathology. http://librepathology.org/wiki/index.php/Neuroendocrine_tumour_of_the_pancreas
![Histopathology of a pancreatic endocrine tumor (insulinoma). Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[5]](/images/2/2f/Pancreatic_insulinoma_histology_2.JPG)
![Histopathology of a pancreatic endocrine tumor (insulinoma). Chromogranin A immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[5]](/images/a/a3/Pancreatic_insulinoma_histopathology_3.JPG)
![Histopathology of a pancreatic endocrine tumor (insulinoma). Insulin immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[5]](/images/d/d5/Pancreatic_insulinoma_histology_4.JPG)