Rosiglitazone
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Rosiglitazone is a Thiazolidinedione that is FDA approved for the treatment of as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Common adverse reactions include Edema, Weight gain, Headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Glycemic control in adults with type 2 diabetes mellitus
- Dosing information
- Starting dosage: 4 mg PO qd or bid
- For patients who respond inadequately following 8 to 12 weeks of treatment, as determined by reduction in fasting plasma glucose (FPG), the dose may be increased to 8 mg daily. Increases in the dose of AVANDIA should be accompanied by careful monitoring for adverse events related to fluid retention. AVANDIA may be taken with or without food.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Rosiglitazone in adult patients.
Non–Guideline-Supported Use
Coronary stent stenosis
- Dosing information
- 4 mg orally daily15505001
Insulin resistance
- Dosing information
- 4 mg daily 14520628
Metabolic syndrome
- Dosing information
- 4 mg daily for 8 weeks14759393
Non-alcoholic fatty liver
- Dosing information
- 4 mg daily for 1 month, then 8 mg daily thereafter18503774
Polycystic ovary syndrome
- Dosing information
- 4 mg twice daily12620440
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Glycemic control in adults with type 2 diabetes mellitus
- Dosing information
- Starting dosage: 4 mg PO qd or bid
- For patients who respond inadequately following 8 to 12 weeks of treatment, as determined by reduction in fasting plasma glucose (FPG), the dose may be increased to 8 mg daily. Increases in the dose of AVANDIA should be accompanied by careful monitoring for adverse events related to fluid retention. AVANDIA may be taken with or without food.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Rosiglitazone in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Rosiglitazone in pediatric patients.
Contraindications
- Initiation of AVANDIA in patients with established New York Heart Association (NYHA) Class III or IV heart failure is contraindicated.
- Use in patients with a history of a hypersensitivity reaction to rosiglitazone or any of the product’s ingredients.
Warnings
Cardiac Failure
AVANDIA, like other thiazolidinediones, alone or in combination with other antidiabetic agents, can cause fluid retention, which may exacerbate or lead to heart failure. Patients should be observed for signs and symptoms of heart failure. If these signs and symptoms develop, the heart failure should be managed according to current standards of care. Furthermore, discontinuation or dose reduction of rosiglitazone must be considered [see Boxed Warning]. Patients with congestive heart failure (CHF) NYHA Class I and II treated with AVANDIA have an increased risk of cardiovascular events. A 52-week, double-blind, placebo-controlled, echocardiographic trial was conducted in 224 patients with type 2 diabetes mellitus and NYHA Class I or II CHF (ejection fraction ≤45%) on background antidiabetic and CHF therapy. An independent committee conducted a blinded evaluation of fluid-related events (including congestive heart failure) and cardiovascular hospitalizations according to predefined criteria (adjudication). Separate from the adjudication, other cardiovascular adverse events were reported by investigators. Although no treatment difference in change from baseline of ejection fractions was observed, more cardiovascular adverse events were observed following treatment with AVANDIA compared with placebo during the 52-week trial. (See Table 1.)
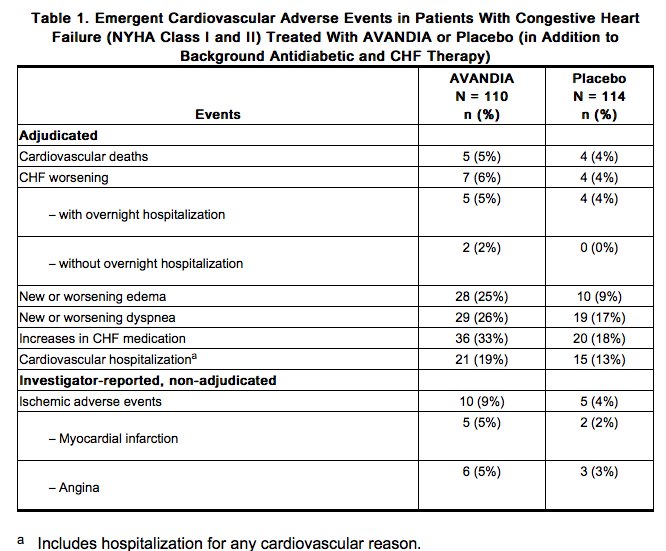
In a long-term, cardiovascular outcome trial (RECORD) in patients with type 2 diabetes [see Adverse Reactions (6.1)], the incidence of heart failure was higher in patients treated with AVANDIA [2.7% (61/2,220) compared with active control 1.3% (29/2,227), HR 2.10 (95% CI: 1.35, 3.27)]. Initiation of AVANDIA in patients with established NYHA Class III or IV heart failure is contraindicated. AVANDIA is not recommended in patients with symptomatic heart failure. Patients experiencing acute coronary syndromes have not been studied in controlled clinical trials. In view of the potential for development of heart failure in patients having an acute coronary event, initiation of AVANDIA is not recommended for patients experiencing an acute coronary event, and discontinuation of AVANDIA during this acute phase should be considered. Patients with NYHA Class III and IV cardiac status (with or without CHF) have not been studied in controlled clinical trials. AVANDIA is not recommended in patients with NYHA Class III and IV cardiac status. Congestive Heart Failure During Coadministration of AVANDIA With Insulin: In trials in which AVANDIA was added to insulin, AVANDIA increased the risk of congestive heart failure. Coadministration of AVANDIA and insulin is not recommended. In 7 controlled, randomized, double-blind trials which had durations from 16 to 26 weeks and which were included in a meta-analysis [see Warnings and Precautions (5.2)], patients with type 2 diabetes mellitus were randomized to coadministration of AVANDIA and insulin (N = 1,018) or insulin (N = 815). In these 7 trials, AVANDIA was added to insulin. These trials included patients with long-standing diabetes (median duration of 12 years) and a high prevalence of pre-existing medical conditions, including peripheral neuropathy, retinopathy, ischemic heart disease, vascular disease, and congestive heart failure. The total number of patients with emergent congestive heart failure was 23 (2.3%) and 8 (1.0%) in the group receiving AVANDIA plus insulin and the insulin group, respectively. Heart Failure in Observational Studies of Elderly Diabetic Patients Comparing AVANDIA to Pioglitazone: Three observational studies in elderly diabetic patients (age 65 years and older) found that AVANDIA statistically significantly increased the risk of hospitalized heart failure compared to use of pioglitazone. One other observational study in patients with a mean age of 54 years, which also included an analysis in a subpopulation of patients >65 years of age, found no statistically significant increase in emergency department visits or hospitalization for heart failure in patients treated with AVANDIA compared to pioglitazone in the older subgroup.
Major Adverse Cardiovascular Events
Data from long-term, prospective, randomized, controlled clinical trials of AVANDIA versus metformin or sulfonylureas, particularly a cardiovascular outcome trial (RECORD), observed no difference in overall mortality or in major adverse cardiovascular events (MACE) and its components. A meta-analysis of mostly short-term trials suggested an increased risk for myocardial infarction with AVANDIA compared with placebo. Cardiovascular Events in Large, Long-term, Prospective, Randomized, Controlled Trials of AVANDIA: RECORD, a prospectively designed cardiovascular outcome trial (mean follow-up 5.5 years; 4,447 patients), compared the addition of AVANDIA to metformin or a sulfonylurea (N = 2,220) with a control group of metformin plus sulfonylurea (N = 2,227) in patients with type 2 diabetes [see Adverse Reactions (6.1)]. Non-inferiority was demonstrated for the primary endpoint, cardiovascular hospitalization or cardiovascular death, for AVANDIA compared with control [HR 0.99 (95% CI: 0.85, 1.16)] demonstrating no overall increased risk in cardiovascular morbidity or mortality. The hazard ratios for total mortality and MACE were consistent with the primary endpoint and the 95% CI similarly excluded a 20% increase in risk for AVANDIA. The hazard ratios for the components of MACE were 0.72 (95% CI: 0.49, 1.06) for stroke, 1.14 (95% CI: 0.80, 1.63) for myocardial infarction, and 0.84 (95% CI: 0.59, 1.18) for cardiovascular death. The results of RECORD are consistent with the findings of 2 earlier long-term, prospective, randomized, controlled clinical trials (each trial >3 years’ duration; total of 9,620 patients) (see Figure 1). In patients with impaired glucose tolerance (DREAM trial), although the incidence of cardiovascular events was higher among subjects who were randomized to AVANDIA in combination with ramipril than among subjects randomized to ramipril alone, no statistically significant differences were observed for MACE and its components between AVANDIA and placebo. In type 2 diabetes patients who were initiating oral agent monotherapy (ADOPT trial), no statistically significant differences were observed for MACE and its components between AVANDIA and metformin or a sulfonylurea.
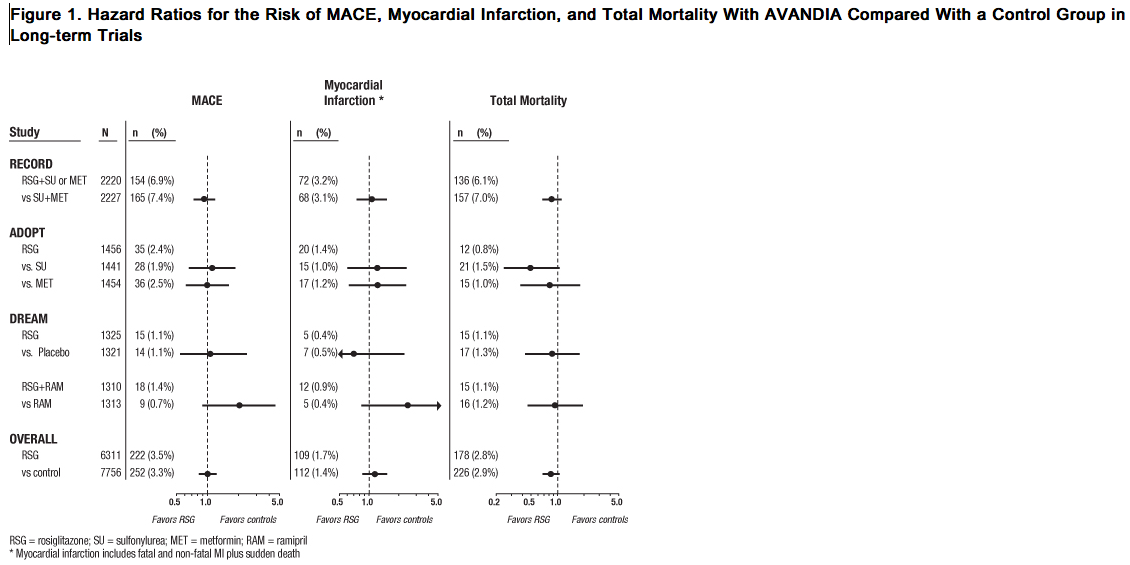
Cardiovascular Events in a Group of 52 Clinical Trials: In a meta-analysis of 52 double-blind, randomized, controlled clinical trials designed to assess glucose-lowering efficacy in type 2 diabetes (mean duration 6 months), a statistically significant increased risk of myocardial infarction with AVANDIA versus pooled comparators was observed [0.4% versus 0.3%; OR 1.8, (95% CI: 1.03, 3.25)]. A statistically non-significant increased risk of MACE was observed with AVANDIA versus pooled comparators (OR 1.44, 95% CI: 0.95, 2.20). In the placebo-controlled trials, a statistically significant increased risk of myocardial infarction [0.4% versus 0.2%, OR 2.23 (95% CI: 1.14, 4.64)] and statistically non-significant increased risk of MACE [0.7% versus 0.5%, OR 1.53 (95% CI: 0.94, 2.54)] with AVANDIA were observed. In the active-controlled trials, there was no increased risk of myocardial infarction or MACE. Mortality in Observational Studies of AVANDIA Compared to Pioglitazone: Three observational studies in elderly diabetic patients (age 65 years and older) found that AVANDIA statistically significantly increased the risk of all-cause mortality compared to use of pioglitazone. One observational study in patients with a mean age of 54 years found no difference in all-cause mortality between patients treated with AVANDIA compared to pioglitazone and reported similar results in the subpopulation of patients >65 years of age. One additional small, prospective, observational study found no statistically significant differences for CV mortality and all-cause mortality in patients treated with AVANDIA compared to pioglitazone.
Edema
AVANDIA should be used with caution in patients with edema. In a clinical trial in healthy volunteers who received 8 mg of AVANDIA once daily for 8 weeks, there was a statistically significant increase in median plasma volume compared with placebo. Since thiazolidinediones, including rosiglitazone, can cause fluid retention, which can exacerbate or lead to congestive heart failure, AVANDIA should be used with caution in patients at risk for heart failure. Patients should be monitored for signs and symptoms of heart failure [see Boxed Warning, Warnings and Precautions (5.1), Patient Counseling Information (17)]. In controlled clinical trials of patients with type 2 diabetes, mild to moderate edema was reported in patients treated with AVANDIA, and may be dose related. Patients with ongoing edema were more likely to have adverse events associated with edema if started on combination therapy with insulin and AVANDIA [see Adverse Reactions (6.1)].
Weight Gain
Dose-related weight gain was seen with AVANDIA alone and in combination with other hypoglycemic agents (Table 2). The mechanism of weight gain is unclear but probably involves a combination of fluid retention and fat accumulation. In postmarketing experience, there have been reports of unusually rapid increases in weight and increases in excess of that generally observed in clinical trials. Patients who experience such increases should be assessed for fluid accumulation and volume-related events such as excessive edema and congestive heart failure.
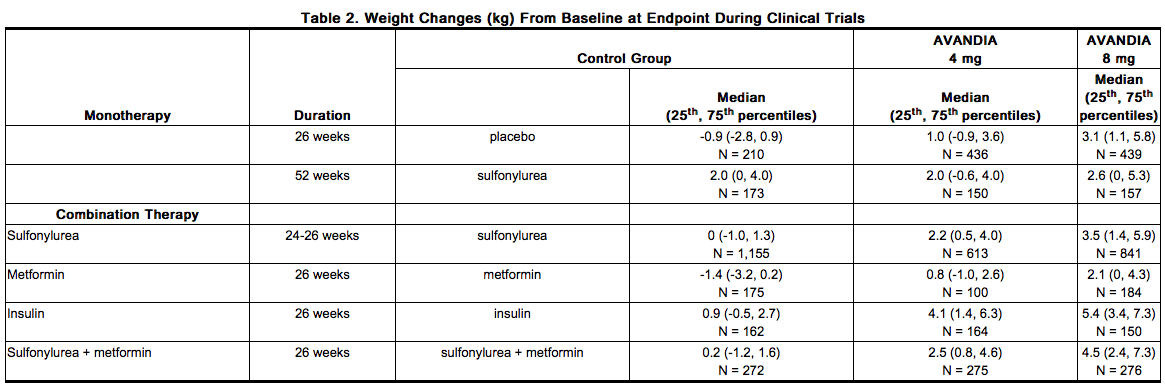
In a 4- to 6-year, monotherapy, comparative trial (ADOPT) in patients recently diagnosed with type 2 diabetes not previously treated with antidiabetic medication [see Clinical Studies (14.1)], the median weight change (25th, 75th percentiles) from baseline at 4 years was 3.5 kg (0.0, 8.1) for AVANDIA, 2.0 kg (-1.0, 4.8) for glyburide, and -2.4 kg (-5.4, 0.5) for metformin. In a 24-week trial in pediatric patients aged 10 to 17 years treated with AVANDIA 4 to 8 mg daily, a median weight gain of 2.8 kg (25th, 75th percentiles: 0.0, 5.8) was reported.
Hepatic Effects
Liver enzymes should be measured prior to the initiation of therapy with AVANDIA in all patients and periodically thereafter per the clinical judgment of the healthcare professional. Therapy with AVANDIA should not be initiated in patients with increased baseline liver enzyme levels (ALT >2.5X upper limit of normal). Patients with mildly elevated liver enzymes (ALT levels ≤2.5X upper limit of normal) at baseline or during therapy with AVANDIA should be evaluated to determine the cause of the liver enzyme elevation. Initiation of, or continuation of, therapy with AVANDIA in patients with mild liver enzyme elevations should proceed with caution and include close clinical follow-up, including liver enzyme monitoring, to determine if the liver enzyme elevations resolve or worsen. If at any time ALT levels increase to >3X the upper limit of normal in patients on therapy with AVANDIA, liver enzyme levels should be rechecked as soon as possible. If ALT levels remain >3X the upper limit of normal, therapy with AVANDIA should be discontinued. If any patient develops symptoms suggesting hepatic dysfunction, which may include unexplained nausea, vomiting, abdominal pain, fatigue, anorexia and/or dark urine, liver enzymes should be checked. The decision whether to continue the patient on therapy with AVANDIA should be guided by clinical judgment pending laboratory evaluations. If jaundice is observed, drug therapy should be discontinued. [See Adverse Reactions (6.2, 6.3).]
Macular Edema
Macular edema has been reported in postmarketing experience in some diabetic patients who were taking AVANDIA or another thiazolidinedione. Some patients presented with blurred vision or decreased visual acuity, but some patients appear to have been diagnosed on routine ophthalmologic examination. Most patients had peripheral edema at the time macular edema was diagnosed. Some patients had improvement in their macular edema after discontinuation of their thiazolidinedione. Patients with diabetes should have regular eye exams by an ophthalmologist, per the Standards of Care of the American Diabetes Association. Additionally, any diabetic who reports any kind of visual symptom should be promptly referred to an ophthalmologist, regardless of the patient’s underlying medications or other physical findings. [See Adverse Reactions (6.1).]
Fractures
Long-term trials (ADOPT and RECORD) show an increased incidence of bone fracture in patients, particularly female patients, taking AVANDIA [see Adverse Reactions (6.1)]. This increased incidence was noted after the first year of treatment and persisted during the course of the trial. The majority of the fractures in the women who received AVANDIA occurred in the upper arm, hand, and foot. These sites of fracture are different from those usually associated with postmenopausal osteoporosis (e.g., hip or spine). Other trials suggest that this risk may also apply to men, although the risk of fracture among women appears higher than that among men. The risk of fracture should be considered in the care of patients treated with AVANDIA, and attention given to assessing and maintaining bone health according to current standards of care.
Hematologic Effects
Decreases in mean hemoglobin and hematocrit occurred in a dose-related fashion in adult patients treated with AVANDIA [see Adverse Reactions (6.2)]. The observed changes may be related to the increased plasma volume observed with treatment with AVANDIA.
Diabetes and Blood Glucose Control
Patients receiving AVANDIA in combination with other hypoglycemic agents may be at risk for hypoglycemia, and a reduction in the dose of the concomitant agent may be necessary. Periodic fasting blood glucose and HbA1c measurements should be performed to monitor therapeutic response.
Ovulation
Therapy with AVANDIA, like other thiazolidinediones, may result in ovulation in some premenopausal anovulatory women. As a result, these patients may be at an increased risk for pregnancy while taking AVANDIA. Thus, adequate contraception in premenopausal women should be recommended. This possible effect has not been specifically investigated in clinical trials; therefore, the frequency of this occurrence is not known. Although hormonal imbalance has been seen in preclinical studies [see Nonclinical Toxicology (13.1)], the clinical significance of this finding is not known. If unexpected menstrual dysfunction occurs, the benefits of continued therapy with AVANDIA should be reviewed.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice. Adult: In clinical trials, approximately 9,900 patients with type 2 diabetes have been treated with AVANDIA. Short-term Trials of AVANDIA as Monotherapy and in Combination With Other Hypoglycemic Agents: The incidence and types of adverse events reported in short-term clinical trials of AVANDIA as monotherapy are shown in Table 3.
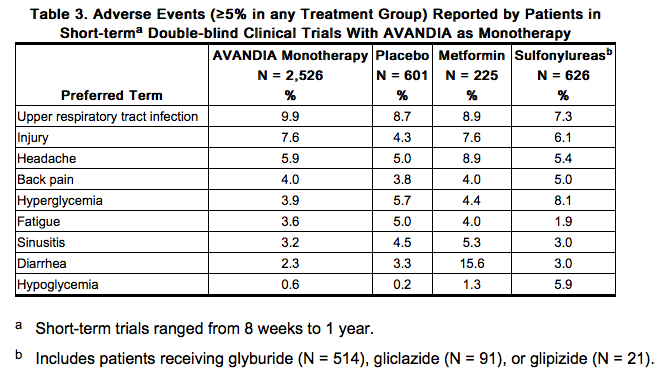
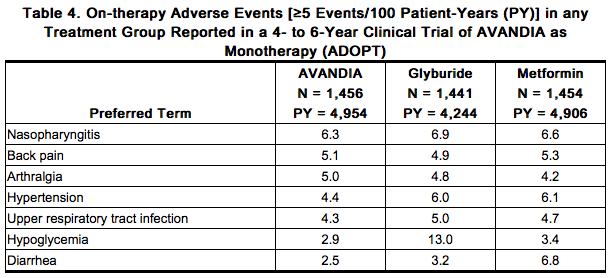
Overall, the types of adverse reactions without regard to causality reported when AVANDIA was used in combination with a sulfonylurea or metformin were similar to those during monotherapy with AVANDIA. Events of anemia and edema tended to be reported more frequently at higher doses, and were generally mild to moderate in severity and usually did not require discontinuation of treatment with AVANDIA. In double-blind trials, anemia was reported in 1.9% of patients receiving AVANDIA as monotherapy compared with 0.7% on placebo, 0.6% on sulfonylureas, and 2.2% on metformin. Reports of anemia were greater in patients treated with a combination of AVANDIA and metformin (7.1%) and with a combination of AVANDIA and a sulfonylurea plus metformin (6.7%) compared with monotherapy with AVANDIA or in combination with a sulfonylurea (2.3%). Lower pre-treatment hemoglobin/hematocrit levels in patients enrolled in the metformin combination clinical trials may have contributed to the higher reporting rate of anemia in these trials [see Adverse Reactions (6.2)]. In clinical trials, edema was reported in 4.8% of patients receiving AVANDIA as monotherapy compared with 1.3% on placebo, 1.0% on sulfonylureas, and 2.2% on metformin. The reporting rate of edema was higher for AVANDIA 8 mg in sulfonylurea combinations (12.4%) compared with other combinations, with the exception of insulin. Edema was reported in 14.7% of patients receiving AVANDIA in the insulin combination trials compared with 5.4% on insulin alone. Reports of new onset or exacerbation of congestive heart failure occurred at rates of 1% for insulin alone, and 2% (4 mg) and 3% (8 mg) for insulin in combination with AVANDIA [see Boxed Warning, Warnings and Precautions (5.1)]. In controlled combination therapy trials with sulfonylureas, mild to moderate hypoglycemic symptoms, which appear to be dose related, were reported. Few patients were withdrawn for hypoglycemia (<1%) and few episodes of hypoglycemia were considered to be severe (<1%). Hypoglycemia was the most frequently reported adverse event in the fixed-dose insulin combination trials, although few patients withdrew for hypoglycemia (4 of 408 for AVANDIA plus insulin and 1 of 203 for insulin alone). Rates of hypoglycemia, confirmed by capillary blood glucose concentration ≤50 mg/dL, were 6% for insulin alone and 12% (4 mg) and 14% (8 mg) for insulin in combination with AVANDIA. [See Warnings and Precautions (5.9).] Long-term Trial of AVANDIA as Monotherapy: A 4- to 6-year trial (ADOPT) compared the use of AVANDIA (n = 1,456), glyburide (n = 1,441), and metformin (n = 1,454) as monotherapy in patients recently diagnosed with type 2 diabetes who were not previously treated with antidiabetic medication. Table 4 presents adverse reactions without regard to causality; rates are expressed per 100 patient-years (PY) exposure to account for the differences in exposure to trial medication across the 3 treatment groups. In ADOPT, fractures were reported in a greater number of women treated with AVANDIA (9.3%, 2.7/100 patient-years) compared with glyburide (3.5%, 1.3/100 patient-years) or metformin (5.1%, 1.5/100 patient-years). The majority of the fractures in the women who received rosiglitazone were reported in the upper arm, hand, and foot. [See Warnings and Precautions (5.7).] The observed incidence of fractures for male patients was similar among the 3 treatment groups.
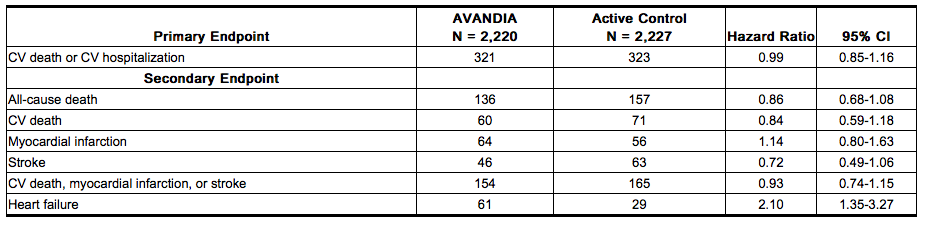
Long-term Trial of AVANDIA as Combination Therapy (RECORD): RECORD (Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycemia in Diabetes) was a multicenter, randomized, open-label, non-inferiority trial in subjects with type 2 diabetes inadequately controlled on maximum doses of metformin or sulfonylurea (glyburide, gliclazide, or glimepiride) to compare the time to reach the combined cardiovascular endpoint of cardiovascular death or cardiovascular hospitalization between patients randomized to the addition of AVANDIA versus metformin or sulfonylurea. The trial included patients who have failed metformin or sulfonylurea monotherapy; those who failed metformin (n = 2,222) were randomized to receive either AVANDIA as add-on therapy (n = 1,117) or add-on sulfonylurea (n = 1,105), and those who failed sulfonylurea (n = 2,225) were randomized to receive either AVANDIA as add-on therapy (n = 1,103) or add-on metformin (n = 1,122). Patients were treated to target HbA1c ≤7% throughout the trial.
The mean age of patients in this trial was 58 years, 52% were male, and the mean duration of follow-up was 5.5 years. AVANDIA demonstrated non-inferiority to active control for the primary endpoint of cardiovascular hospitalization or cardiovascular death (HR 0.99, 95% CI: 0.85-1.16). There were no significant differences between groups for secondary endpoints with the exception of congestive heart failure (see Table 5). The incidence of congestive heart failure was significantly greater among patients randomized to AVANDIA.
There was an increased incidence of bone fracture for subjects randomized to AVANDIA in addition to metformin or sulfonylurea compared with those randomized to metformin plus sulfonylurea (8.3% versus 5.3%) [see Warnings and Precautions (5.7)]. The majority of fractures were reported in the upper limbs and distal lower limbs. The risk of fracture appeared to be higher in females relative to control (11.5% versus 6.3%), than in males relative to control (5.3% versus 4.3%). Additional data are necessary to determine whether there is an increased risk of fracture in males after a longer period of follow-up.
Pediatric: AVANDIA has been evaluated for safety in a single, active-controlled trial of pediatric patients with type 2 diabetes in which 99 were treated with AVANDIA and 101 were treated with metformin. The most common adverse reactions (>10%) without regard to causality for either AVANDIA or metformin were headache (17% versus 14%), nausea (4% versus 11%), nasopharyngitis (3% versus 12%), and diarrhea (1% versus 13%). In this trial, one case of diabetic ketoacidosis was reported in the metformin group. In addition, there were 3 patients in the rosiglitazone group who had FPG of approximately 300 mg/dL, 2+ ketonuria, and an elevated anion gap.
-Laboratory Abnormalities
Hematologic: Decreases in mean hemoglobin and hematocrit occurred in a dose-related fashion in adult patients treated with AVANDIA (mean decreases in individual trials as much as 1.0 g/dL hemoglobin and as much as 3.3% hematocrit). The changes occurred primarily during the first 3 months following initiation of therapy with AVANDIA or following a dose increase in AVANDIA. The time course and magnitude of decreases were similar in patients treated with a combination of AVANDIA and other hypoglycemic agents or monotherapy with AVANDIA. Pre-treatment levels of hemoglobin and hematocrit were lower in patients in metformin combination trials and may have contributed to the higher reporting rate of anemia. In a single trial in pediatric patients, decreases in hemoglobin and hematocrit (mean decreases of 0.29 g/dL and 0.95%, respectively) were reported. Small decreases in hemoglobin and hematocrit have also been reported in pediatric patients treated with AVANDIA. White blood cell counts also decreased slightly in adult patients treated with AVANDIA. Decreases in hematologic parameters may be related to increased plasma volume observed with treatment with AVANDIA. Lipids: Changes in serum lipids have been observed following treatment with AVANDIA in adults [see Clinical Pharmacology (12.2)]. Small changes in serum lipid parameters were reported in children treated with AVANDIA for 24 weeks. Serum Transaminase Levels: In pre-approval clinical trials in 4,598 patients treated with AVANDIA (3,600 patient-years of exposure) and in a long-term 4- to 6-year trial in 1,456 patients treated with AVANDIA (4,954 patient-years exposure), there was no evidence of drug-induced hepatotoxicity. In pre-approval controlled trials, 0.2% of patients treated with AVANDIA had elevations in ALT >3X the upper limit of normal compared with 0.2% on placebo and 0.5% on active comparators. The ALT elevations in patients treated with AVANDIA were reversible. Hyperbilirubinemia was found in 0.3% of patients treated with AVANDIA compared with 0.9% treated with placebo and 1% in patients treated with active comparators. In pre-approval clinical trials, there were no cases of idiosyncratic drug reactions leading to hepatic failure. [See Warnings and Precautions (5.5).] In the 4- to 6-year ADOPT trial, patients treated with AVANDIA (4,954 patient-years exposure), glyburide (4,244 patient-years exposure), or metformin (4,906 patient-years exposure), as monotherapy, had the same rate of ALT increase to >3X upper limit of normal (0.3 per 100 patient-years exposure). In the RECORD trial, patients randomized to AVANDIA in addition to metformin or sulfonylurea (10,849 patient-years exposure) and to metformin plus sulfonylurea (10,209 patient-years exposure) had a rate of ALT increase to ≥3X upper limit of normal of approximately 0.2 and 0.3 per 100 patient-years exposure, respectively.
Postmarketing Experience
In addition to adverse reactions reported from clinical trials, the events described below have been identified during post-approval use of AVANDIA. Because these events are reported voluntarily from a population of unknown size, it is not possible to reliably estimate their frequency or to always establish a causal relationship to drug exposure. In patients receiving thiazolidinedione therapy, serious adverse events with or without a fatal outcome, potentially related to volume expansion (e.g., congestive heart failure, pulmonary edema, and pleural effusions) have been reported [see Boxed Warning, Warnings and Precautions (5.1)]. There are postmarketing reports with AVANDIA of hepatitis, hepatic enzyme elevations to 3 or more times the upper limit of normal, and hepatic failure with and without fatal outcome, although causality has not been established. There are postmarketing reports with AVANDIA of rash, pruritus, urticaria, angioedema, anaphylactic reaction, Stevens-Johnson syndrome [see Contraindications (4)], and new onset or worsening diabetic macular edema with decreased visual acuity.
Drug Interactions
There is limited information regarding Rosiglitazone Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Rosiglitazone in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Rosiglitazone in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Rosiglitazone during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Rosiglitazone in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Rosiglitazone in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Rosiglitazone in geriatric settings.
Gender
There is no FDA guidance on the use of Rosiglitazone with respect to specific gender populations.
Race
There is no FDA guidance on the use of Rosiglitazone with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Rosiglitazone in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Rosiglitazone in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Rosiglitazone in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Rosiglitazone in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Rosiglitazone Administration in the drug label.
Monitoring
There is limited information regarding Rosiglitazone Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Rosiglitazone and IV administrations.
Overdosage
There is limited information regarding Rosiglitazone overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Rosiglitazone Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Rosiglitazone Mechanism of Action in the drug label.
Structure
There is limited information regarding Rosiglitazone Structure in the drug label.
Pharmacodynamics
There is limited information regarding Rosiglitazone Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Rosiglitazone Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Rosiglitazone Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Rosiglitazone Clinical Studies in the drug label.
How Supplied
There is limited information regarding Rosiglitazone How Supplied in the drug label.
Storage
There is limited information regarding Rosiglitazone Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Rosiglitazone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Rosiglitazone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Rosiglitazone Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Rosiglitazone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Rosiglitazone Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Rosiglitazone Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.