Rift valley fever
Template:DiseaseDisorder infobox
|
WikiDoc Resources for Rift valley fever |
|
Articles |
|---|
|
Most recent articles on Rift valley fever Most cited articles on Rift valley fever |
|
Media |
|
Powerpoint slides on Rift valley fever |
|
Evidence Based Medicine |
|
Cochrane Collaboration on Rift valley fever |
|
Clinical Trials |
|
Ongoing Trials on Rift valley fever at Clinical Trials.gov Trial results on Rift valley fever Clinical Trials on Rift valley fever at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Rift valley fever NICE Guidance on Rift valley fever
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Rift valley fever Discussion groups on Rift valley fever Patient Handouts on Rift valley fever Directions to Hospitals Treating Rift valley fever Risk calculators and risk factors for Rift valley fever
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Rift valley fever |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Rift Valley fever (RVF) is an acute, fever-causing viral disease that affects domestic animals (such as cattle, buffalo, sheep, goats, and camels) and humans. RVF is most commonly associated with mosquito-borne epidemics during years of unusually heavy rainfall. The disease was first reported among livestock in Kenya around 1915, but the virus was not isolated until 1931. RVF outbreaks occur across sub-Saharan Africa, with outbreaks occurring elsewhere infrequently (but sometimes severely - in Egypt in 1977-78, several million people were infected and thousands died during a violent epidemic. In Kenya in 1998, the virus claimed the lives of over 400 Kenyans. In September 2000 an outbreak was confirmed in Saudi Arabia and Yemen).
Related Key Words and Synonyms:
RVF infection
Epidemiology and Demographics
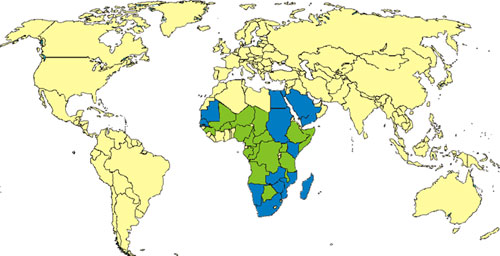
Where is the disease found?
RVF is generally found in regions of eastern and southern Africa where sheep and cattle are raised, but the virus also exists in most countries of sub-Saharan Africa and in Madagascar. In September 2000, a RVF outbreak was reported in Saudi Arabia and subsequently Yemen. These cases represent the first Rift Valley fever cases identified outside Africa.
RVF virus primarily affects livestock and can cause disease in a large number of domestic animals (this situation is referred to as an "epizootic"). The presence of an RVF epizootic can lead to an epidemic among humans who are exposed to diseased animals. The most notable epizootic of RVF, which occurred in Kenya in 1950-1951, resulted in the death of an estimated 100,000 sheep. In 1977, the virus was detected in Egypt (probably exported there in infected domestic animals from Sudan) and caused a large outbreak of RVF among animals and humans. The first epidemic of RVF in West Africa was reported in 1987 and was linked to construction of the Senegal River Project. The project caused flooding in the lower Senegal River area and altered interactions between animals and humans resulting in transmission of the RVF virus to humans.
How do humans get RVF?
Humans usually get RVF through bites from infected mosquitoes and possibly other biting insects that have virus-contaminated mouthparts. Humans can also get the disease if they are exposed to the blood, body fluids, or tissues of infected animals. Direct exposure to infected animals can occur during slaughter or through veterinary and obstetric procedures. Infection through aerosol transmission of RVF virus has occurred in the laboratory environment.
Risk Factors
Studies have shown that sleeping outdoors at night in geographical regions where outbreaks occur could be a risk factor for exposure to mosquito and other insect vectors. Animal herdsmen, abattoir workers, and other individuals who work with animals in RVF-endemic areas (areas where the virus is present) have an increased risk for infection. Persons in high-risk professions, such as veterinarians and slaughterhouse workers, have an increased chance of contracting the virus from an infected animal. International travelers increase their chances of getting the disease when they visit RVF-endemic locations during periods when sporadic cases or epidemics are occurring.
Pathophysiology & Etiology
The disease is caused by the RVF virus, a member of the genus Phlebovirus in the family Bunyaviridae.
Natural History
The disease was first reported among livestock by veterinary officers in Kenya in the early 1900s.
Rift Valley Fever Outbreak --- Kenya, November 2006--January 2007
In mid-December 2006, several unexplained fatalities associated with fever and generalized bleeding were reported to the Kenya Ministry of Health (KMOH) from Garissa District in North Eastern Province (NEP). By December 20, a total of 11 deaths had been reported. Of serum samples collected from the first 19 patients, Rift Valley fever (RVF) virus RNA or immunoglobulin M (IgM) antibodies against RVF virus were found in samples from 10 patients; all serum specimens were negative for yellow fever, Ebola, Crimean-Congo hemorrhagic fever, and dengue viruses. The outbreak was confirmed by isolation of RVF virus from six of the specimens. Humans can be infected with RVF virus from bites of mosquitoes or other arthropod vectors that have fed on animals infected with RVF virus, or through contact with viremic animals, particularly livestock. Reports of livestock deaths and unexplained animal abortions in NEP provided further evidence of an RVF outbreak. On December 20, an investigation was launched by KMOH, the Kenya Field Epidemiology and Laboratory Training Program (FELTP), the Kenya Medical Research Institute (KEMRI), the Walter Reed Project of the U.S. Army Medical Research Unit, CDC-Kenya's Global Disease Detection Center, and other partners, including the World Health Organization (WHO) and Médecins Sans Frontières (MSF). This report describes the findings from that initial investigation and the control measures taken in response to the RVF outbreak, which spread to multiple additional provinces and districts, resulting in 404 cases with 118 deaths as of January 25, 2007.
Teams of investigators conducted patient interviews and reviewed medical records from December 1 forward in major health-care facilities in the districts from which cases were first reported. The teams detected additional cases by meeting with elders, other leaders, and health-care providers in villages where cases had been reported and in adjacent villages. Blood samples from patients with suspected RVF were collected and maintained at 39.2ºF (4.0ºC). Samples from NEP and surrounding areas were transported to a field laboratory established at Garissa Provincial Hospital by CDC, KEMRI, and KMOH; samples from other areas were sent to KEMRI laboratories in Nairobi and to a laboratory in Malindi that was supported by a team from Health Canada.
A suspected case was defined as acute onset of fever (>99.5ºF [>37.5ºC]) with headache or muscle and joint pain since December 1 in a person who had no other known cause of acute febrile illness (e.g., malaria). A probable case was defined as acute onset of fever in a person with unexplained bleeding (i.e., in stool, vomit, or sputum or from gums, nose, vagina, skin, or eyes), vision deterioration, or altered consciousness. A confirmed case was defined as a suspected or probable case with laboratory confirmation of the presence in serum of anti-RVF virus IgM by enzyme-linked immunosorbent assay (ELISA) or RVF virus RNA by reverse transcription--polymerase chain reaction (RT-PCR).
The index case was reported in Garissa District in a patient who had symptom onset on November 30, 2006. Retrospective analysis of sera collected during July--November 2006 at Garissa Provincial Hospital revealed no evidence of earlier acute RVF infections. As of January 25, 2007, a total of 404 cases of RVF had been reported in Kenya with 118 deaths, a case-fatality rate of 29%. Of the reported cases, 115 (29%) were laboratory confirmed by anti-RVF virus IgM by ELISA (64 cases, 56%) or RT-PCR (79, 69%), including 28 cases (24%) confirmed by both. Of the remaining 289 cases, 109 were classified as probable.
Of the 230 patients with available demographic information, 140 (61%) were male. Patients ranged in age from 4 to 85 years, with a median age of 27 years (30 years for females and 25 years for males). RVF cases were reported from three districts in NEP (Garissa [175 cases], Ijara [125], and Wajir [26]); five districts in Coast Province (Kilifi [38], Tana River [16], Malindi [eight], Isiolo [eight], and Taita Taveta [one]); two districts in Central Province (Kirinyanga [two] and Maragua [one]); one district in Rift Valley Province (Kajiado [three]); and one from Nairobi Area (Figure 2). The patient from Nairobi had traveled to NEP during the week before illness onset but was hospitalized in Nairobi. Ijara (population 79,932) and Garissa (population 420,918) districts had the highest RVF incidence rates: 156 and 42 per 100,000 population, respectively.
Among the first 97 reported cases from Garissa and Wajir districts with detailed epidemiologic information available, 71 (73%) met the probable case definition; 38 of the 62 patients who provided blood samples tested positive by IgM ELISA, RT-PCR, or both. The most frequently reported symptoms among the 97 patients were fever (100%), headache (90%), bleeding (76%), malaise (70%), muscle pain (62%), back pain (60%), vomiting (56%), and joint pain (51%).
Two thirds of the 66 patients who provided information on potential risk factors reported having an animal that was recently ill. The most frequently reported RVF risk factors during the 2 weeks preceding illness onset were drinking unboiled (raw) milk (72%); living within 100 meters of a swamp (70%); having an ill animal (67%); drinking milk from an ill animal (59%); working as a herdsman (50%); having a dead animal (50%); and slaughtering an animal (42%). Approximately 9% of patients reported contact with another ill human.
The outbreak peaked on December 24, 2006, and the number of daily cases has been declining since December 27, 2006. A ban on livestock slaughtering in Garissa District went into effect on December 27 and was expanded as RVF was detected in additional districts. Vaccination of animals with live, attenuated RVF vaccine began on January 8, 2007. Prevention messages were developed in three languages (English, Kiswhali, and Somali), and public meetings (known as barazas) were held to spread information rapidly to the community. Messages also were disseminated via radio, a widely used communication medium in NEP. Village elders, chiefs, and religious leaders were consulted throughout Garissa District, leading to a district ban on the slaughter of livestock and closure of the livestock market. Health-care workers were trained to care for persons suspected to be infected with RVF virus.
2000-2001: Rift Valley Fever Outbreak in Saudi Arabia and Yemen
In September 2000, the Ministry of Health of the Kingdom of Saudi Arabia, and subsequently the Ministry of Health of Yemen received reports of unexplained hemorrhagic fever in humans and associated animal deaths from the southwestern border of Saudi Arabia and Yemen. CDC confirmed the outbreak to be caused by Rift Valley fever virus.
Rift Valley Fever -- East Africa, 1997-1998
In December 1997, the Kenya Ministry of Health and the World Health Organization (WHO) in Nairobi received reports of 478 unexplained deaths in the North Eastern province of Kenya and southern Somalia. Clinical features included acute onset of fever and headache associated with hemorrhage (hematochezia, hematemesis, and bleeding from other mucosal sites). Local health officials also reported high rates of illness and death resulting from hemorrhage among domestic animals in the area. This report describes the preliminary results of the outbreak investigation and the results of a serologic survey.
From late October 1997 through January 1998, torrential rains occurred in most of East Africa, resulting in the worst flooding in the region since 1961 and rainfall that was 60-100 times the seasonal average (National Climatic Data Center, unpublished data, 1998). Diagnostic testing of the initial 36 specimens received at the National Institute of Virology, South Africa, and at CDC confirmed acute infection with Rift Valley fever (RVF) virus in 17 (47%) persons from whom specimens were obtained; confirmation was made by detection of IgM antibodies, virus isolation, reverse-transcriptase-polymerase chain reaction for viral nucleic acid, or immunohistochemistry.
Active surveillance conducted by WHO, the Kenya Ministry of Health, and international relief organizations during December 22-28 in 18 villages (population: 200,000) in Garissa district, North Eastern province, Kenya, identified 170 deaths resulting from a "bleeding disease." Severe flooding and large distances between settlements complicated case ascertainment and subsequent evaluation. Despite these constraints, the surveillance system received reports and blood specimens for 231 cases of unexplained severe febrile illness with onset from November 25, 1997, through February 14, 1998. Of the 231 reported cases, 115 met the case definition for hemorrhagic fever (i.e., fever and mucosal or gastrointestinal bleeding). Of the 115 patients with hemorrhagic fever, 58% were male (median age: 30 years {range: 3-85 years}); diagnostic testing demonstrated acute RVF viral infection in 27 (23%) (Figure_1). Of the 116 persons whose illnesses did not meet the case-definition for hemorrhagic fever, 26 (22%) had acute infection with RVF virus. Of these 26 persons, 14 had symptoms compatible with complications of RVF viral infection, including nine with neurologic disease and five with visual disturbances. In addition to the confirmed RVF cases in the North Eastern province and the Gedo, Hiran, and Lower Shabeelle provinces of Somalia, acute confirmed RVF cases were identified in the Central (one case), Eastern (nine cases), and Rift Valley (12 cases) provinces of Kenya (Figure_2).
Studies conducted during this outbreak included human, livestock, and entomologic sampling. Using a multistage cluster sampling strategy based on the population distribution in Garissa district, an international task force led by the Kenya Ministry of Health conducted a cross-sectional study to examine risk factors and determine the prevalence of recent infection with RVF virus. Anti-RVF virus IgM was detected by enzyme-linked immunosorbent assay in 18 (9%) of the 202 persons in the sample; all 18 had recently been ill, compared with 80% of the seronegative persons (p=0.05). The study did not identify statistically significant differences in the frequency of IgM antibody by sex or age. However, contact with livestock (e.g., herding, milking, slaughtering, and sheltering animals in the home) was statistically associated with serologic evidence of acute infection with RVF virus (p less than 0.01).
In this cross-sectional survey, livestock owners reported losses of approximately 70% of their sheep and goats and 20%-30% of cattle and camels. Other infections contributing to the high mortality in the epizootic included nonspecific pneumonia, pasteurellosis, contagious caprine pleuropneumonia, contagious pustular dermatitis, bluetongue, and complications of mange and foot rot (Field Mission of the Food and Agriculture Organization of the United Nations, unpublished data, 1998). RVF serologic results from animal samples collected by veterinary staff in this and other regions of Kenya are pending.
In February 1998 in Garissa district, 3180 mosquitoes from three trapping sites were collected. Three of the nine captured species have been previously implicated in RVF transmission (Anopheles coustani, Mansonia africana, and M. uniformis). Viral isolation studies are under way.
Diagnosis
History and Symptoms
In humans the virus can cause several different syndromes. Usually sufferers have either no symptoms or only a mild illness with fever, headache, myalgia and liver abnormalities. In a small percentage of cases (< 2%) the illness can progress to hemorrhagic fever syndrome, meningoencephalitis (inflammation of the brain), or affecting the eye. Patients who become ill usually experience fever, generalized weakness, back pain, dizziness, and weight loss at the onset of the illness. Typically, patients recover within 2-7 days after onset.
Approximately 1% of human sufferers die of the disease. Amongst livestock the fatality level is significantly higher. In pregnant livestock infected with RVF there is the abortion of virtually 100% of fetuses. An epizootic (animal disease epidemic) of RVF is usually first indicated by a wave of unexplained abortions.
How do humans get RVF?
Humans usually get RVF through bites from infected mosquitoes and possibly other biting insects that have virus-contaminated mouthparts. Humans can also get the disease if they are exposed to the blood, body fluids, or tissues of infected animals. Direct exposure to infected animals can occur during slaughter or through veterinary and obstetric procedures. Infection through aerosol transmission of RVF virus has occurred in the laboratory environment.
Risk Stratification and Prognosis
Are there complications after recovery?
The most common complication associated with RVF is inflammation of the retina (a structure connecting the nerves of the eye to the brain). As a result, approximately 1% - 10% of affected patients may have some permanent vision loss.
Is the disease ever fatal?
Approximately 1% of humans that become infected with RVF die of the disease. Case-fatality proportions are significantly higher for infected animals. The most severe impact is observed in pregnant livestock infected with RVF, which results in abortion of virtually 100% of fetuses.
Treatment
There is no established course of treatment for patients infected with RVF virus.
Pharmacotherapy
Studies in monkeys and other animals have shown promise for ribavirin, an antiviral drug, for future use in humans. Additional studies suggest that interferon, immune modulators, and convalescent-phase plasma may also help in the treatment of patients with RVF.
Primary Prevention
A person's chances of becoming infected can be reduced by taking measures to decrease contact with mosquitoes and other bloodsucking insects through the use of mosquito repellents and bednets. Avoiding exposure to blood or tissues of animals that may potentially be infected is an important protective measure for persons working with animals in RVF-endemic areas.
Measures to prevent bites from mosquitoes, ticks, fleas and other insects and arthropods:
To reduce the possibility of being bitten by insects or arthropods that can transmit diseases (vector-borne), such as malaria, dengue, and tickborne encephalitis (TBE), you should―
- Use an insect repellent on exposed skin to repel mosquitoes, ticks, fleas and other arthropods. EPA-registered repellents include products containing DEET (N,N-diethylmetatoluamide) and picaridin (KBR 3023). DEET concentrations of 30% to 50% are effective for several hours. Picaridin, available at 7% and 15 % concentrations, needs more frequent application.
- DEET formulations as high as 50% are recommended for both adults and children over 2 months of age. Protect infants less than 2 months of age by using a carrier draped with mosquito netting with an elastic edge for a tight fit.
- When using sunscreen, apply sunscreen first and then repellent. Repellent should be washed off at the end of the day before going to bed.
- Wear long-sleeved shirts which should be tucked in, long pants, and hats to cover exposed skin. When you visit areas with ticks and fleas, wear boots, not sandals, and tuck pants into socks.
- Inspect your body and clothing for ticks during outdoor activity and at the end of the day. Wear light-colored or white clothing so ticks can be more easily seen. Removing ticks right away can prevent some infections.
- Apply permethrin-containing (e.g., Permanone) or other insect repellents to clothing, shoes, tents, mosquito nets, and other gear for greater protection. Permethrin is not labeled for use directly on skin. Most repellent is generally removed from clothing and gear by a single washing, but permethrin-treated clothing is effective for up to 5 washings.
- Be aware that mosquitoes that transmit malaria are most active during twilight periods (dawn and dusk or in the evening).
- Stay in air-conditioned or well-screened housing, and/ or sleep under an insecticide treated bed net. Bed nets should be tucked under mattresses and can be sprayed with a repellent if not already treated with an insecticide.
- Daytime biters include mosquitoes that transmit dengue and chikungunya viruses and sand flies that transmit leishmaniasis.
Acknowledgements
The content on this page was first contributed by: C. Michael Gibson, M.S., M.D.
List of contributors:
Pilar Almonacid
References
External links
Template:Viral diseases Template:SIB ar:حمى الوادي المتصدع de:Rifttalfieber fa:تب دره ریفت it:Febbre della Rift Valley nl:Riftdalkoorts wa:Five del Vå do Rift