Ribavirin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
RISK OF SERIOUS DISORDERS AND RIBAVIRIN-ASSOCIATED EFFECTS
See full prescribing information for complete Boxed Warning.
Ribavirin monotherapy is not effective for the treatment of chronic hepatitis C virus infection and should not be used alone for this indication. The primary clinical toxicity of ribavirin is hemolytic anemia. The anemia associated with ribavirin therapy may result in worsening of cardiac disease and lead to fatal and nonfatal myocardial infarctions. Patients with a history of significant or unstable cardiac disease should not be treated with ribavirin.
Significant teratogenic and/or embryocidal effects have been demonstrated in all animal species exposed to ribavirin. In addition, ribavirin has a multiple dose half-life of 12 days, and it may persist in non-plasma compartments for as long as 6 months. Therefore, ribavirin, including ribavirin tablets, is contraindicated in women who are pregnant and in the male partners of women who are pregnant. Extreme care must be taken to avoid pregnancy during therapy and for 6 months after completion of therapy in both female patients and in female partners of male patients who are taking ribavirin therapy. At least two reliable forms of effective contraception must be utilized during treatment and during the 6 month post treatment follow-up period:
|
Overview
Ribavirin is a nucleoside analogue that is FDA approved for the treatment of chronic hepatitis C (CHC) virus infection in combination with peginterferon alfa-2a in adults with compensated liver disease not previously treated with interferon alpha, and in CHC patients coinfected with HIV (1). There is a Black Box Warning for this drug as shown here. Common adverse reactions include injection site reaction, pruritus, weight decrease, diarrhea, gastrointestinal symptoms, loss of appetite, nausea, vomiting, neutropenia, asthenia, dizziness, excluding vertigo, headache, insomnia, fatigue and influenza-like illness..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Chronic Hepatitis C Monoinfection
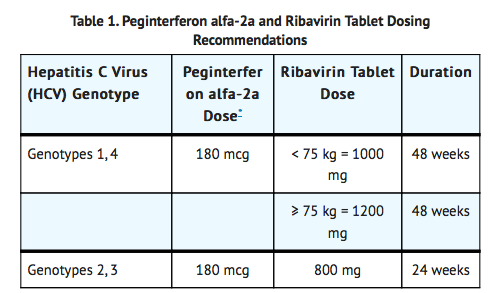
- Dosage: 800-1200 mg/day, administered in two divided doses, for 24-48 week. Administer with food.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ribavirin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ribavirin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Ribavirin FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ribavirin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ribavirin in pediatric patients.
Contraindications
There is limited information regarding Ribavirin Contraindications in the drug label.
Warnings
|
RISK OF SERIOUS DISORDERS AND RIBAVIRIN-ASSOCIATED EFFECTS
See full prescribing information for complete Boxed Warning.
Ribavirin monotherapy is not effective for the treatment of chronic hepatitis C virus infection and should not be used alone for this indication. The primary clinical toxicity of ribavirin is hemolytic anemia. The anemia associated with ribavirin therapy may result in worsening of cardiac disease and lead to fatal and nonfatal myocardial infarctions. Patients with a history of significant or unstable cardiac disease should not be treated with ribavirin.
Significant teratogenic and/or embryocidal effects have been demonstrated in all animal species exposed to ribavirin. In addition, ribavirin has a multiple dose half-life of 12 days, and it may persist in non-plasma compartments for as long as 6 months. Therefore, ribavirin, including ribavirin tablets, is contraindicated in women who are pregnant and in the male partners of women who are pregnant. Extreme care must be taken to avoid pregnancy during therapy and for 6 months after completion of therapy in both female patients and in female partners of male patients who are taking ribavirin therapy. At least two reliable forms of effective contraception must be utilized during treatment and during the 6 month post treatment follow-up period:
|
There is limited information regarding Ribavirin Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Ribavirin Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Ribavirin Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Ribavirin Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Ribavirin in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ribavirin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ribavirin during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Ribavirin in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Ribavirin in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Ribavirin in geriatric settings.
Gender
There is no FDA guidance on the use of Ribavirin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ribavirin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Ribavirin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Ribavirin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ribavirin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ribavirin in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Ribavirin Administration in the drug label.
Monitoring
There is limited information regarding Ribavirin Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Ribavirin and IV administrations.
Overdosage
There is limited information regarding Ribavirin overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Ribavirin Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Ribavirin Mechanism of Action in the drug label.
Structure
There is limited information regarding Ribavirin Structure in the drug label.
Pharmacodynamics
There is limited information regarding Ribavirin Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Ribavirin Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Ribavirin Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Ribavirin Clinical Studies in the drug label.
How Supplied
There is limited information regarding Ribavirin How Supplied in the drug label.
Storage
There is limited information regarding Ribavirin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Ribavirin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Ribavirin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Ribavirin Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Ribavirin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Ribavirin Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Ribavirin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]
Overview
Ribavirin (Copegus®; Rebetol®; Ribasphere®; Vilona®,Virazole®, also generics from Sandoz, Teva, Warrick) is an anti-viral drug which is active against a number of DNA and RNA viruses. It is a member of the nucleosideantimetabolite drugs that interfere with duplication of viral genetic material. Though not effective against all viruses, ribavirin is remarkable as a small molecule for its wide range of activity, including important activities against influenzas, flaviviruses and agents of many viral hemorrhagic fevers.
Category
Antiviral
US Brand Names
Copegus®, Pegetron®, Rebetol®, Rebetron®, Ribasphere®, Virazole®
FDA Package Insert
Description | Clinical Pharmacology | Microbiology | Indications and Usage | Contraindications | Warnings and Precautions | Adverse Reactions | Drug Interactions | Overdosage | Clinical Studies | Dosage and Administration | How Supplied | Labels and Packages
Mechanism of Action
The mechanism by which ribavirin contributes to its antiviral efficacy in the clinic is not fully understood. Ribavirin has direct antiviral activity in tissue culture against many RNA viruses. Ribavirin increases the mutation frequency in the genomes of several viruses and ribavirin triphosphate inhibits HCV polymerase in a biochemical reaction.