Quinapril tablet: Difference between revisions
Ahmed Zaghw (talk | contribs) No edit summary |
Ahmed Zaghw (talk | contribs) No edit summary |
||
| Line 198: | Line 198: | ||
In patients undergoing major surgery or during anesthesia with agents that produce [[hypotension]], Accupril will block [[angiotensin II]] formation secondary to compensatory [[renin]] release. If hypotension occurs and is considered to be due to this mechanism, it can be corrected by volume expansion. | In patients undergoing major surgery or during anesthesia with agents that produce [[hypotension]], Accupril will block [[angiotensin II]] formation secondary to compensatory [[renin]] release. If hypotension occurs and is considered to be due to this mechanism, it can be corrected by volume expansion. | ||
|clinicalTrials======Hypertension===== | |clinicalTrials======Hypertension===== | ||
:* Accupril has been evaluated for safety in 4960 subjects and patients. Of these, 3203 patients, including 655 elderly patients, participated in controlled clinical trials. Accupril has been evaluated for long-term safety in over 1400 patients treated for 1 year or more. | :* Accupril has been evaluated for safety in 4960 subjects and patients. Of these, 3203 patients, including 655 elderly patients, participated in controlled clinical trials. Accupril has been evaluated for long-term safety in over 1400 patients treated for 1 year or more. | ||
:* Adverse experiences were usually mild and transient. | :* Adverse experiences were usually mild and transient. | ||
:* In placebo-controlled trials, discontinuation of therapy because of adverse events was required in 4.7% of patients with [[hypertension]]. | :* In placebo-controlled trials, discontinuation of therapy because of adverse events was required in 4.7% of patients with [[hypertension]]. | ||
| Line 212: | Line 209: | ||
=====Heart Failure===== | =====Heart Failure===== | ||
:*Accupril has been evaluated for safety in 1222 Accupril treated patients. Of these, 632 patients participated in controlled clinical trials. In placebo-controlled trials, discontinuation of therapy because of adverse events was required in 6.8% of patients with [[congestive heart failure]]. | :*Accupril has been evaluated for safety in 1222 Accupril treated patients. Of these, 632 patients participated in controlled clinical trials. In placebo-controlled trials, discontinuation of therapy because of adverse events was required in 6.8% of patients with [[congestive heart failure]]. | ||
| Line 224: | Line 220: | ||
=====General===== | =====General===== | ||
:*back pain, [[malaise]], viral infections, [[anaphylactoid reaction]] | :*back pain, [[malaise]], viral infections, [[anaphylactoid reaction]]. | ||
=====Cardiovascular===== | =====Cardiovascular===== | ||
Revision as of 18:54, 22 April 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2], Amr Marawan, M.D. [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
FETAL TOXICITY
See full prescribing information for complete Boxed Warning.
:* When pregnancy is detected, discontinue ACCUPRIL as soon as possible.
|
Overview
Quinapril tablet is an Angiotensin converting enzyme inhibitor that is FDA approved for the {{{indicationType}}} of hypertension, heart failure, angioedema in black patients. There is a Black Box Warning for this drug as shown here. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
- Accupril is contraindicated in patients who are hypersensitive to this product and in patients with a history of angioedema related to previous treatment with an ACE inhibitor.
- Do not co-administer aliskiren with Accupril in patients with diabetes.
Warnings
|
FETAL TOXICITY
See full prescribing information for complete Boxed Warning.
:* When pregnancy is detected, discontinue ACCUPRIL as soon as possible.
|
Anaphylactoid and Possibly Related Reactions
Presumably because angiotensin-converting inhibitors affect the metabolism of eicosanoids and polypeptides, including endogenous bradykinin, patients receiving ACE inhibitors (including Accupril) may be subject to a variety of adverse reactions, some of them serious.
Head and Neck Angioedema
Angioedema of the face, extremities, lips, tongue, glottis, and larynx has been reported in patients treated with ACE inhibitors and has been seen in 0.1% of patients receiving Accupril.
In two similarly sized U.S. postmarketing trials that, combined, enrolled over 3,000 black patients and over 19,000 non-blacks, angioedema was reported in 0.30% and 0.55% of blacks (in study 1 and 2 respectively) and 0.39% and 0.17% of non-blacks.
Angioedema associated with laryngeal edema can be fatal. If laryngeal stridor or angioedema of the face, tongue, or glottis occurs, treatment with Accupril should be discontinued immediately, the patient treated in accordance with accepted medical care, and carefully observed until the swelling disappears. In instances where swelling is confined to the face and lips, the condition generally resolves without treatment; antihistamines may be useful in relieving symptoms. Where there is involvement of the tongue, glottis, or larynx likely to cause airway obstruction, emergency therapy including, but not limited to, subcutaneous epinephrine solution 1:1000 (0.3 to 0.5 mL) should be promptly administered.
Patients taking concomitant mTOR inhibitor (e.g. temsirolimus) therapy may be at increased risk for angioedema.
Intestinal Angioedema
Intestinal angioedema has been reported in patients treated with ACE inhibitors. These patients presented with abdominal pain (with or without nausea or vomiting); in some cases there was no prior history of facial angioedema and C-1 esterase levels were normal. The angioedema was diagnosed by procedures including abdominal CT scan or ultrasound, or at surgery, and symptoms resolved after stopping the ACE inhibitor. Intestinal angioedema should be included in the differential diagnosis of patients on ACE inhibitors presenting with abdominal pain.
Patients with a history of angioedema
Patients with a history of angioedema unrelated to ACE inhibitor therapy may be at increased risk of angioedema while receiving an ACE inhibitor.
Anaphylactoid reactions during desensitization
Two patients undergoing desensitizing treatment with hymenoptera venom while receiving ACE inhibitors sustained life-threatening anaphylactoid reactions. In the same patients, these reactions were avoided when ACE inhibitors were temporarily withheld, but they reappeared upon inadvertent rechallenge.
Anaphylactoid reactions during membrane exposure
Anaphylactoid reactions have been reported in patients dialyzed with high-flux membranes and treated concomitantly with an ACE inhibitor. Anaphylactoid reactions have also been reported in patients undergoing low-density lipoprotein apheresis with dextran sulfate absorption.
Hepatic Failure
Rarely, ACE inhibitors have been associated with a syndrome that starts with cholestatic jaundice and progresses to fulminant hepatic necrosis and (sometimes) death. The mechanism of this syndrome is not understood. Patients receiving ACE inhibitors who develop jaundice or marked elevations of hepatic enzymes should discontinue the ACE inhibitor and receive appropriate medical follow-up.
Hypotension
Excessive hypotension is rare in patients with uncomplicated hypertension treated with Accupril alone. Patients with heart failure given Accupril commonly have some reduction in blood pressure, but discontinuation of therapy because of continuing symptomatic hypotension usually is not necessary when dosing instructions are followed. Caution should be observed when initiating therapy in patients with heart failure. In controlled studies, syncope was observed in 0.4% of patients (N=3203); this incidence was similar to that observed for captopril (1%) and enalapril (0.8%).
Patients at risk of excessive hypotension, sometimes associated with oliguria and/or progressive azotemia, and rarely with acute renal failure and/or death, include patients with the following conditions or characteristics: heart failure, hyponatremia, high dose diuretic therapy, recent intensive diuresis or increase in diuretic dose, renal dialysis, or severe volume and/or salt depletion of any etiology. It may be advisable to eliminate the diuretic (except in patients with heart failure), reduce the diuretic dose or cautiously increase salt intake (except in patients with heart failure) before initiating therapy with Accupril in patients at risk for excessive hypotension who are able to tolerate such adjustments.
In patients at risk of excessive hypotension, therapy with Accupril should be started under close medical supervision. Such patients should be followed closely for the first two weeks of treatment and whenever the dose of Accupril and/or diuretic is increased. Similar considerations may apply to patients with ischemic heart or cerebrovascular disease in whom an excessive fall in blood pressure could result in a myocardial infarction or a cerebrovascular accident.
If excessive hypotension occurs, the patient should be placed in the supine position and, if necessary, receive an intravenous infusion of normal saline. A transient hypotensive response is not a contraindication to further doses of Accupril, which usually can be given without difficulty once the blood pressure has stabilized. If symptomatic hypotension develops, a dose reduction or discontinuation of Accupril or concomitant diuretic may be necessary.
Neutropenia/Agranulocytosis
Another ACE inhibitor, captopril, has been shown to cause agranulocytosis and bone marrow depression rarely in patients with uncomplicated hypertension, but more frequently in patients with renal impairment, especially if they also have a collagen vascular disease, such as systemic lupus erythematosus or scleroderma. Agranulocytosis did occur during Accupril treatment in one patient with a history of neutropenia during previous captopril therapy. Available data from clinical trials of Accupril are insufficient to show that, in patients without prior reactions to other ACE inhibitors, Accupril does not cause agranulocytosis at similar rates. As with other ACE inhibitors, periodic monitoring of white blood cell counts in patients with collagen vascular disease and/or renal disease should be considered.
Fetal Toxicity
Pregnancy Category D
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Accupril as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus.
In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examinations to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue Accupril, unless it is considered life-saving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Closely observe infants with histories of in utero exposure to Accupril for hypotension, oliguria, and hyperkalemia.
No teratogenic effects of Accupril were seen in studies of pregnant rats and rabbits. On a mg/kg basis, the doses used were up to 180 times (in rats) and one time (in rabbits) the maximum recommended human dose.
Precautions
General
Impaired renal function
As a consequence of inhibiting the renin-angiotensin-aldosterone system, changes in renal function may be anticipated in susceptible individuals. In patients with severe heart failure whose renal function may depend on the activity of the renin-angiotensin-aldosterone system, treatment with ACE inhibitors, including Accupril, may be associated with oliguria and/or progressive azotemia and rarely acute renal failure and/or death.
In clinical studies in hypertensive patients with unilateral or bilateral renal artery stenosis, increases in blood urea nitrogen and serum creatinine have been observed in some patients following ACE inhibitor therapy. These increases were almost always reversible upon discontinuation of the ACE inhibitor and/or diuretic therapy. In such patients, renal function should be monitored during the first few weeks of therapy.
Some patients with hypertension or heart failure with no apparent preexisting renal vascular disease have developed increases in blood urea and serum creatinine, usually minor and transient, especially when Accupril has been given concomitantly with a diuretic. This is more likely to occur in patients with preexisting renal impairment. Dosage reduction and/or discontinuation of any diuretic and/or Accupril may be required.
Evaluation of patients with hypertension or heart failure should always include assessment of renal function.
Hyperkalemia and potassium-sparing diuretics
In clinical trials, hyperkalemia (serum potassium ≥5.8 mmol/L) occurred in approximately 2% of patients receiving Accupril. In most cases, elevated serum potassium levels were isolated values which resolved despite continued therapy. Less than 0.1% of patients discontinued therapy due to hyperkalemia. Risk factors for the development of hyperkalemia include renal insufficiency, diabetes mellitus, and the concomitant use of potassium-sparing diuretics, potassium supplements, and/or potassium-containing salt substitutes, which should be used cautiously, if at all, with Accupril.
Cough
Presumably due to the inhibition of the degradation of endogenous bradykinin, persistent non-productive cough has been reported with all ACE inhibitors, always resolving after discontinuation of therapy. ACE inhibitor-induced cough should be considered in the differential diagnosis of cough.
Surgery/anesthesia
In patients undergoing major surgery or during anesthesia with agents that produce hypotension, Accupril will block angiotensin II formation secondary to compensatory renin release. If hypotension occurs and is considered to be due to this mechanism, it can be corrected by volume expansion.
Adverse Reactions
Clinical Trials Experience
Hypertension
- Accupril has been evaluated for safety in 4960 subjects and patients. Of these, 3203 patients, including 655 elderly patients, participated in controlled clinical trials. Accupril has been evaluated for long-term safety in over 1400 patients treated for 1 year or more.
- Adverse experiences were usually mild and transient.
- In placebo-controlled trials, discontinuation of therapy because of adverse events was required in 4.7% of patients with hypertension.
- Adverse experiences probably or possibly related to therapy or of unknown relationship to therapy occurring in 1% or more of the 1563 patients in placebo-controlled hypertension trials who were treated with Accupril are shown below:
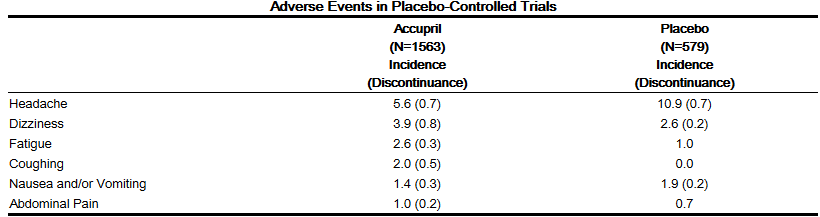
Heart Failure
- Accupril has been evaluated for safety in 1222 Accupril treated patients. Of these, 632 patients participated in controlled clinical trials. In placebo-controlled trials, discontinuation of therapy because of adverse events was required in 6.8% of patients with congestive heart failure.
- Adverse experiences probably or possibly related or of unknown relationship to therapy occurring in 1% or more of the 585 patients in placebo-controlled congestive heart failure trials who were treated with Accupril are shown below:
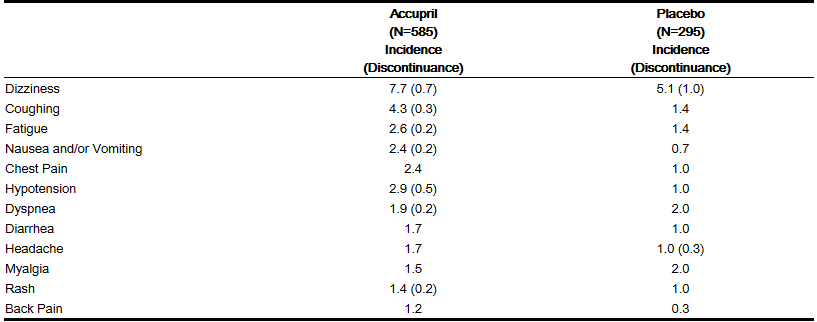
Hypertension and/or Heart Failure
- Clinical adverse experiences probably, possibly, or definitely related, or of uncertain relationship to therapy occurring in 0.5% to 1.0% (except as noted) of the patients with CHF or hypertension treated with Accupril (with or without concomitant diuretic) in controlled or uncontrolled trials (N=4847) and less frequent, clinically significant events seen in clinical trials or post-marketing experience (the rarer events are in italics) include (listed by body system):
General
- back pain, malaise, viral infections, anaphylactoid reaction.
Cardiovascular
Hematology
Gastrointestinal
- Flatulence, dry mouth or throat, constipation, gastrointestinal hemorrhage, pancreatitis, abnormal liver function tests, dyspepsia
Nervous/Psychiatric
Integumentary
- Alopecia, increased sweating, pemphigus, pruritus, exfoliative dermatitis, photosensitivity reaction, dermatopolymyositis
Urogenital
- urinary tract infection, impotence, acute renal failure, worsening renal failure
Respiratory
Other
Angioedema
- Angioedema has been reported in patients receiving Accupril (0.1%). Angioedema associated with laryngeal edema may be fatal. If angioedema of the face, extremities, lips, tongue, glottis, and/or larynx occurs, treatment with Accupril should be discontinued and appropriate therapy instituted immediately.
Clinical Laboratory Test Findings
Hematology
Hyperkalemia
Creatinine and Blood Urea Nitrogen
- Increases (>1.25 times the upper limit of normal) in serum creatinine and blood urea nitrogen were observed in 2% and 2%, respectively, of all patients treated with Accupril alone. Increases are more likely to occur in patients receiving concomitant diuretic therapy than in those on Accupril alone. These increases often remit on continued therapy. In controlled studies of heart failure, increases in blood urea nitrogen and serum creatinine were observed in 11% and 8%, respectively, of patients treated with Accupril; most often these patients were receiving diuretics with or without digitalis.
Postmarketing Experience
There is limited information regarding Quinapril tablet Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Quinapril tablet Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Quinapril tablet in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Quinapril tablet in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Quinapril tablet during labor and delivery.
Nursing Mothers
Nursing Mothers
Because Accupril is secreted in human milk, caution should be exercised when this drug is administered to a nursing woman.
Pediatric Use
Pediatric Use
Neonates with a history of in utero exposure to Accupril
If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function. Removal of Accupril, which crosses the placenta, from the neonatal circulation is not significantly accelerated by these means.
The safety and effectiveness of Accupril in pediatric patients have not been established.
Geriatic Use
Geriatric Use
Clinical studies of Accupril did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Elderly patients exhibited increased area under the plasma concentration time curve and peak levels for quinaprilat compared to values observed in younger patients; this appeared to relate to decreased renal function rather than to age itself.
Gender
There is no FDA guidance on the use of Quinapril tablet with respect to specific gender populations.
Race
There is no FDA guidance on the use of Quinapril tablet with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Quinapril tablet in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Quinapril tablet in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Quinapril tablet in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Quinapril tablet in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Quinapril tablet Administration in the drug label.
Monitoring
There is limited information regarding Quinapril tablet Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Quinapril tablet and IV administrations.
Overdosage
There is limited information regarding Quinapril tablet overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Quinapril tablet Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Quinapril tablet Mechanism of Action in the drug label.
Structure
There is limited information regarding Quinapril tablet Structure in the drug label.
Pharmacodynamics
There is limited information regarding Quinapril tablet Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Quinapril tablet Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Quinapril tablet Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Quinapril tablet Clinical Studies in the drug label.
How Supplied
There is limited information regarding Quinapril tablet How Supplied in the drug label.
Storage
There is limited information regarding Quinapril tablet Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Quinapril tablet |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Quinapril tablet |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Quinapril tablet Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Quinapril tablet interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Quinapril tablet Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Quinapril tablet Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.