Q fever: Difference between revisions
| Line 27: | Line 27: | ||
==[[Q fever laboratory tests|Lab Tests]]== | ==[[Q fever laboratory tests|Lab Tests]]== | ||
== Risk Stratification and Prognosis== | == Risk Stratification and Prognosis== | ||
Revision as of 14:57, 2 February 2012
For patient information click here
| Q fever | |
 | |
|---|---|
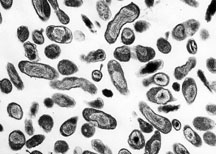
| Organism Responsible for Q fever, Rocky Mountain Laboratories, NIAID, NIH | |
| ICD-10 | A78 |
| ICD-9 | 083.0 |
| MeSH | D011778 |
|
Q fever Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Q fever On the Web |
|
American Roentgen Ray Society Images of Q fever |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Epidemiology & Demographics
Pathophysiology
History & Symptoms
Diagnosis
Lab Tests
Risk Stratification and Prognosis
Coxiella burnetii is a highly infectious agent that is rather resistant to heat and drying. It can become airborne and inhaled by humans. A single C. burnetii organism may cause disease in a susceptible person. This agent could be developed for use in biological warfare and is considered a potential terrorist threat.
Treatment
Acute Pharmacotherapies
Doxycycline is the treatment of choice for acute Q fever. Antibiotic treatment is most effective when initiated within the first 3 days of illness. A dose of 100 mg of doxycycline taken orally twice daily for 15-21 days is a frequently prescribed therapy. Quinolone antibiotics have demonstrated good in vitro activity against C. burnetii and may be considered by the physician. Therapy should be started again if the disease relapses.
Q fever in pregnancy is especially difficult to treat because doxycycline and ciprofloxacin are contraindicated in pregnancy. The preferred treatment is five weeks of co-trimoxazole.[1]
Chronic Pharmacotherapies
Chronic Q fever endocarditis is much more difficult to treat effectively and often requires the use of multiple drugs. Two different treatment protocols have been evaluated: 1) doxycycline in combination with quinolones for at least 4 years and 2) doxycycline in combination with hydroxychloroquine for 1.5 to 3 years. The second therapy leads to fewer relapses, but requires routine eye exams to detect accumulation of chloroquine. Surgery to remove damaged valves may be required for some cases of C. burnetii endocarditis.
Prevention

In the United States, Q fever outbreaks have resulted mainly from occupational exposure involving veterinarians, meat processing plant workers, sheep and dairy workers, livestock farmers, and researchers at facilities housing sheep. Prevention and control efforts should be directed primarily toward these groups and environments.
The following measures should be used in the prevention and control of Q fever:
- Educate the public on sources of infection.
- Appropriately dispose of placenta, birth products, fetal membranes, and aborted fetuses at facilities housing sheep and goats.
- Restrict access to barns and laboratories used in housing potentially infected animals.
- Use only pasteurized milk and milk products.
- Use appropriate procedures for bagging, autoclaving, and washing of laboratory clothing.
- Vaccinate (where possible) individuals engaged in research with pregnant sheep or live C. burnetii.
- Quarantine imported animals.
- Ensure that holding facilities for sheep should be located away from populated areas. Animals should be routinely tested for antibodies to C. burnetii, and measures should be implemented to prevent airflow to other occupied areas.
- Counsel persons at highest risk for developing chronic Q fever, especially persons with pre-existing cardiac valvular disease or individuals with vascular grafts.
A vaccine for Q fever has been developed and has successfully protected humans in occupational settings in Australia. However, this vaccine is not commercially available in the United States. Persons wishing to be vaccinated should first have a skin and blood test to determine a history of previous exposure. Individuals who have previously been exposed to C. burnetii should not receive the vaccine because severe reactions, localized to the area of the injected vaccine, may occur. After a single dose of vaccine, protective immunity lasts for many years and revaccination is not generally required. Annual screening is typically recommended.[2] A vaccine for use in animals has also been developed, but it is not available in the United States.
Q fever is effectively prevented by intradermal vaccination using a vaccine composed of killed Coxiella burnetii organisms. A skin and blood test, prior to vaccination must be undertaken in order to establish whether there is pre-existing immunity, as vaccination of immune subjects can result in a severe local reaction. In 2001, Australia introduced a national Q fever vaccination program for people working in "at risk" occupations.
Other
Because of its route of infection it can be used as biological warfare agent. See also bioterrorism.Q-fever is category "B" agent. It is highly contagious and very stable in aerosols in a wide range of temperatures. Just 1-2 particles are enough to infect an individual. Q-fever microorganisms may survive on surfaces up to 60 days (like sporulating bacteria) and C. burnetii is known to reproduce and grow well in chicken egg embryos reaching very high concentrations. Protection against disease is offered by Q-Vax, a whole cell inactivated vaccine developed by a leading Australian vaccine manufacturing company CSL. (http://www.csl.com.au/QFever.asp)
History
It was first described by Edward Holbrook Derrick in abattoir workers in Brisbane, Queensland, Australia. The "Q" stands for “query” and was applied historically at a time when the causative agent was unknown.
In 1937 the bacterium was isolated by Frank Macfarlane Burnet and Mavis Freeman from one of Derrick’s patients for the first time and identified as Rickettsia-species. H.R. Cox and Davis isolated the pathogen from ticks in Montana, USA in 1938, called it Rickettsia diasporica, it was considered nonpathogenic until laboratory investigators were infected; it was officially named Coxiella burnetii the same year. It is a zoonotic disease and most common animal reservoirs are cattle, sheep and goats. Coxiella burnetii is no longer regarded as closely related to Rickettsiae.
Acknowledgements
List of contributors:
Pilar Almonacid
References
http://www.cdc.gov/ncidod/diseases/submenus/sub_q_fever.htm http://www.cdc.gov/ncidod/dvrd/qfever/index.htm http://www.cdc.gov/healthypets/diseases/qfever.htm
- ↑ Carcopino X, Raoult D, Bretelle F, Boubli L, Stein A (2007). "Managing Q fever during pregnancy: The benefits of long-term Cctrimoxazole therapy". Clin Infect Dis. 45: 548&ndash, 555.
Template:Bacterial diseases
cs:Q-horečka
de:Q-Fieber
hr:Q groznica
it:Febbre Q
he:קדחת Q
nl:Q-koorts
fi:Q-kuume