Physostigmine: Difference between revisions
m (Protected "Physostigmine": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
No edit summary |
||
| (13 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{DrugProjectFormSinglePage | ||
| | |authorTag={{AP}} | ||
| | |genericName=Physostigmine | ||
| | |aOrAn=a | ||
| | |drugClass=central nervous system agent, [[cholinesterase inhibitor]] and anti [[glaucoma]] | ||
| | |indicationType=treatment | ||
| | |indication=clinical or toxic dosages of drugs capable of producing the [[anticholinergic syndrome]] | ||
| | |adverseReactions=[[diaphoresis]], abnormal defecation ([[diarrhea]]), excessive [[salivation]] and [[nausea]] and [[vomiting]] | ||
| | |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | ||
| | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | ||
| | |fdaLIADAdult======Past Anesthesia Care===== | ||
| | *Dosage: 0.5 to 1.0 mg intramuscularly or intravenously. IV administration should be at allow controlled rate of no more than 1 mg per minute. Dosage may be repeated at intervals of 10 to 30 minutes if desired patient response is not obtained. | ||
| | |||
| | =====Clinical or Toxic Dosages of Drugs Capable of Producing the Anticholinergic Syndrome===== | ||
| | *Case report of a 68-year-old male patient admitted to the ICU for treatment of a severe thioridazine intoxication<ref name="pmid9366781">{{cite journal| author=Schmidt W, Lang K| title=Life-threatening dysrhythmias in severe thioridazine poisoning treated with physostigmine and transient atrial pacing. | journal=Crit Care Med | year= 1997 | volume= 25 | issue= 11 | pages= 1925-30 | pmid=9366781 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9366781 }} </ref>. | ||
| | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Physostigmine in adult patients. | ||
| | |offLabelAdultNoGuideSupport======Reversal of Postoperative Reactions to Scopolamine===== | ||
| | *Dosage: 1.0-1.2 milligrams IV<ref name="pmid4796567">{{cite journal| author=Holzgrafe RE, Vondrell JJ, Mintz SM| title=Reversal of postoperative reactions to scopolamine with physostigmine. | journal=Anesth Analg | year= 1973 | volume= 52 | issue= 6 | pages= 921-5 | pmid=4796567 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=4796567 }} </ref>. Unique dose. | ||
}} | |||
=====Reversal of Postoperative Reactions to Scopolamine in Post-Partum===== | |||
*Clinical trial of 5 postpartum patients<ref name="pmid4707533">{{cite journal| author=Smiler BG, Bartholomew EG, Sivak BJ, Alexander GD, Brown EM| title=Physostigmine reversal of scopolamine delirium in obstetric patients. | journal=Am J Obstet Gynecol | year= 1973 | volume= 116 | issue= 3 | pages= 326-9 | pmid=4707533 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=4707533 }} </ref>. | |||
|fdaLIADPed======Clinical or Toxic Dosages of Drugs Capable of Producing the Anticholinergic Syndrome===== | |||
*Case report of a 27 month-old patient with amitriptyline overdose<ref name="pmid9366781">{{cite journal| author=Schmidt W, Lang K| title=Life-threatening dysrhythmias in severe thioridazine poisoning treated with physostigmine and transient atrial pacing. | journal=Crit Care Med | year= 1997 | volume= 25 | issue= 11 | pages= 1925-30 | pmid=9366781 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9366781 }} </ref>. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Physostigmine in pediatric patients. | |||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Physostigmine in pediatric patients. | |||
|contraindications=*Physostigmine Salicylate Injection should not be used in the presence of [[asthma]], [[gangrene]], [[diabetes]], [[cardiovascular disease]], [[mechanical obstruction]] of the intestine or urogenital tract or any [[vagotonic]] state, and in patients receiving [[choline esters]] and depolarizing neuromuscular blocking agents ([[decamethonium]], [[succinylcholine]]). | |||
*For post-anesthesia, the concomitant use of [[atropine]] with physostigmine salicylate is not recommended, since the atropine antagonizes the action of physostigmine. | |||
|warnings=*Contains [[sodium bisulfite]], a sulfite that may cause allergic-type reactions including [[anaphylactic symptoms]] and life-threatening or less severe [[asthmatic episodes]] in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in non-asthmatic people. | |||
*If excessive symptoms of [[salivation]], [[emesis]], urination and defecation occur, the use of Physostigmine Salicylate Injection should be terminated. If excessive sweating or [[nausea]] occur, the dosage should be reduced. | |||
*Intravenous administration should be at a slow, controlled rate, no more than 1 mg per minute. Rapid administration can cause [[bradycardia]], [[hypersalivation]] leading to a respiratory difficulties and possible [[convulsions]]. An overdosage of Physostigmine Salicylate Injection can cause a [[cholinergic crisis]]. | |||
|clinicalTrials======Cardiovascular Effects===== | |||
*[[Asystole]] | |||
*[[Bradyarrhythmia]] | |||
**Case Report of [[Alzheimer]] patients being treated with Physostigmine<ref name="pmid8422658">{{cite journal| author=Sano M, Bell K, Marder K, Stricks L, Stern Y, Mayeux R| title=Safety and efficacy of oral physostigmine in the treatment of Alzheimer disease. | journal=Clin Neuropharmacol | year= 1993 | volume= 16 | issue= 1 | pages= 61-9 | pmid=8422658 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8422658 }} </ref> | |||
**Case Report of a patient treated with physostigmine for post-operative anesthesia with [[Scopolamine]]<ref name="pmid4796567">{{cite journal| author=Holzgrafe RE, Vondrell JJ, Mintz SM| title=Reversal of postoperative reactions to scopolamine with physostigmine. | journal=Anesth Analg | year= 1973 | volume= 52 | issue= 6 | pages= 921-5 | pmid=4796567 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=4796567 }} </ref>. | |||
*[[Cardiac dysrhythmia]] | |||
*[[Hypertension]] | |||
**Case report of a 72 year-old patient treated with 0.5 mg q2h for [[Alzheimer's disease]]<ref name="pmid3717434">{{cite journal| author=Cain JW| title=Hypertension associated with oral administration of physostigmine in a patient with Alzheimer's disease. | journal=Am J Psychiatry | year= 1986 | volume= 143 | issue= 7 | pages= 910-2 | pmid=3717434 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3717434 }} </ref>. | |||
*Increased [[cardiac output]]<ref name="pmid6614520">{{cite journal| author=Nilsson E, Meretoja OA, Neuvonen P| title=Hemodynamic responses to physostigmine in patients with a drug overdose. | journal=Anesth Analg | year= 1983 | volume= 62 | issue= 10 | pages= 885-8 | pmid=6614520 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=6614520 }} </ref> | |||
*Increased [[pulmonary arterial wedge pressure]]<ref name="pmid6614520">{{cite journal| author=Nilsson E, Meretoja OA, Neuvonen P| title=Hemodynamic responses to physostigmine in patients with a drug overdose. | journal=Anesth Analg | year= 1983 | volume= 62 | issue= 10 | pages= 885-8 | pmid=6614520 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=6614520 }} </ref> | |||
*[[Tachycardia]] | |||
**Dosage: 2mg may cause tachycardia<ref name="pmid3080707">{{cite journal| author=Janowsky DS, Risch SC, Kennedy B, Ziegler MG, Huey LY| title=Acute effects of physostigmine and neostigmine in man. | journal=Mil Med | year= 1986 | volume= 151 | issue= 1 | pages= 48-51 | pmid=3080707 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3080707 }} </ref> | |||
{{ | =====Endocrine/Metabolic Effects===== | ||
*Increased leves of [[growth hormone]] | |||
**Dosage: 12.5 mg/kg<ref name="pmid8206111">{{cite journal| author=Mazza E, Ghigo E, Boffano G, Valetto M, Maccario M, Arvat E et al.| title=Effects of direct and indirect acetylcholine receptor agonists on growth hormone secretion in humans. | journal=Eur J Pharmacol | year= 1994 | volume= 254 | issue= 1-2 | pages= 17-20 | pmid=8206111 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8206111 }} </ref>. | |||
=====Gastrointestinal Effects===== | |||
== | *Abnormal [[defecation]]: [[diarrhea]] | ||
*Excessive [[salivation]] | |||
*[[Hyperperistalsis]] | |||
*[[Nausea and vomiting]] | |||
== | =====Immunological Effects===== | ||
*[[Hypersensitivity reactions]] | |||
== | =====Musculoeskeletal Effects===== | ||
*Muscle [[fasciculation]] | |||
*[[Muscle weakness]] | |||
=====Neurological Effects===== | |||
*[[Seizures]] | |||
*Dosage: May appear if doses surpass1 mg/min in adults or 0.5 mg/min in children<ref name="pmid1173100">{{cite journal| author=Newton RW| title=Physostigmine salicylate in the treatment of tricyclic antidepressant overdosage. | journal=JAMA | year= 1975 | volume= 231 | issue= 9 | pages= 941-3 | pmid=1173100 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=1173100 }} </ref>. | |||
=====Psychiatric Depression===== | |||
*[[Depression]] | |||
=====Renal Effects===== | |||
*[[Polyuria]] | |||
*[[Urinary incontinence]] | |||
=====Respiratory Effects===== | |||
*[[Bronchospasm]] | |||
*Excessive [[bronchial secretion]]<ref name="pmid363335">{{cite journal| author=Nattel S, Bayne L, Ruedy J| title=Physostigmine in coma due to drug overdose. | journal=Clin Pharmacol Ther | year= 1979 | volume= 25 | issue= 1 | pages= 96-102 | pmid=363335 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=363335 }} </ref> | |||
|useInPregnancyFDA=*Safe use in pregnancy and lactation has not been established; therefore, use in pregnant women, nursing mothers or women who may become pregnant requires that possible benefits be weighed against possible hazards to mother and child. | |||
|AUSPregCat=C | |||
|useInPregnancyAUS=*Safe use in pregnancy and lactation has not been established; therefore, use in pregnant women, nursing mothers or women who may become pregnant requires that possible benefits be weighed against possible hazards to mother and child. | |||
|useInPed=*Recommended dosage is 0.02 mg/kg; intramuscularly or by slow intravenous injection, no more than 0.5 mg per minute. If the toxic effects persist, and there is no sign of cholinergic effects, the dosage may be repeated at 5 to 10 minute intervals until a therapeutic effect is obtained or a maximum of 2 mg dosage is attained. | |||
|administration=*IV administration should be at allow controlled rate of no more than 1 mg per minute. Dosage may be repeated at intervals of 10 to 30 minutes if desired patient response is not obtained. | |||
|overdose=Can cause a cholinergic crisis. Appropriate antidote is atropine sulfate. | |||
|drugBox={{Drugbox2 | |||
| Watchedfields = changed | |||
| verifiedrevid = 464206065 | |||
| IUPAC_name = (3a''R'',8a''S'')-1,3a,8-Trimethyl-1''H'',2''H'',3''H'',3a''H'',8''H'',8a''H''-pyrrolo[2,3-''b'']indol-5-yl ''N''-methylcarbamate | |||
| image = Physostigmine Molecular Structure.png | |||
<!--Clinical data--> | |||
| tradename = Antilirium | |||
| Drugs.com = {{drugs.com|monograph|antilirium}} | |||
| pregnancy_AU = C | |||
| pregnancy_US = C | |||
| pregnancy_category = | |||
| legal_US = Rx-only | |||
| legal_status = | |||
| routes_of_administration = intravenous, intramuscular, ophthalmic | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = Major metabolite: [[Eseroline]] | |||
| elimination_half-life = | |||
== | <!--Identifiers--> | ||
| CASNo_Ref = {{cascite|changed|??}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 57-47-6 | |||
| ATC_prefix = S01 | |||
| ATC_suffix = EB05 | |||
| ATC_supplemental = {{ATC|V03|AB19}} | |||
| PubChem = 5983 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00981 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 5763 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 9U1VM840SP | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D00196 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 27953 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 94 | |||
{{ | <!--Chemical data--> | ||
{{ | | C=15 | H=21 | N=3 | O=2 | ||
| molecular_weight = 275.346 g/mol | |||
[[ | | smiles = O=C(Oc1cc2c(cc1)N([C@H]3N(CC[C@@]23C)C)C)NC | ||
[[ | | InChI = 1/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | ||
| InChIKey = PIJVFDBKTWXHHD-HIFRSBDPBN | |||
[[ | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
[[ | | StdInChI = 1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | ||
[[ | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| StdInChIKey = PIJVFDBKTWXHHD-HIFRSBDPSA-N | |||
}} | |||
|mechAction=Physostigmine Salicylate Injection is a reversible [[anticholinesterase]] which effectively increases the concentration of [[acetylcholine]] at the sites of [[cholinergic transmission]]. The action of [[acetylcholine]] is normally very transient because of its hydrolysis by the enzyme, [[acetylcholinesterase]]. Physostigmine Salicylate Injection inhibits the destructive action of [[acetylcholinesterase]] and thereby prolongs and exaggerates the effect of the [[acetylcholine]]. | |||
|PK=*Physostigmine Salicylate Injection contains a tertiary amine and easily penetrates the [[blood brain barrier]], while an [[anticholinesterase]], such as [[neostigmine]], which has a quaternary ammonium ion is not capable of crossing the barrier. Physostigmine Salicylate Injection can reverse both central and [[peripheral anticholinergia]]. The [[anticholinergic syndrome]] has both central and peripheral signs and symptoms. Central toxic effects include anxiety, [[delirium]], [[disorientation]], [[hallucinations]], [[hyperactivity]] and [[seizures]]. Severe poisoning may produce [[coma]], [[medullary paralysis]] and [[death]]. Peripheral toxicity is characterized by [[tachycardia]], [[hyperpyrexia]], [[mydriasis]], [[vasodilation]], [[urinary retention]], diminution of gastrointestinal motility, decrease of secretion in salivary and sweat glands, and loss of secretions in the pharynx, bronchi, and nasal passages. | |||
*Dramatic reversal of the effects of anticholinergic symptoms can be expected in minutes after the intravenous administration of Physostigmine Salicylate Injection, if the diagnosis is correct and the patient has not suffered anoxia or other insult. The duration of action of Physostigmine Salicylate Injection is relatively short, approximately 45 to 60 minutes. | |||
|howSupplied=*2 mL Ampules packed 10 per box, 1 mg per mL. | |||
|storage=*Store at 20° to 25°C (68° to 77°F) | |||
|packLabel=[[file:FDA label.png|none|600px]] | |||
[[file:Physost Package3.png|none|400px]] | |||
[[file:Physos Package4.png|none|400px]] | |||
|alcohol=Alcohol-Physostigmine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
|brandNames=*[[Antilirium]] | |||
*[[Isopto Eserine]] | |||
}} | |||
{{LabelImage | |||
|fileName=Packagejns.png | |||
}} | |||
Latest revision as of 17:14, 24 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Physostigmine is a central nervous system agent, cholinesterase inhibitor and anti glaucoma that is FDA approved for the treatment of clinical or toxic dosages of drugs capable of producing the anticholinergic syndrome. Common adverse reactions include diaphoresis, abnormal defecation (diarrhea), excessive salivation and nausea and vomiting.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Past Anesthesia Care
- Dosage: 0.5 to 1.0 mg intramuscularly or intravenously. IV administration should be at allow controlled rate of no more than 1 mg per minute. Dosage may be repeated at intervals of 10 to 30 minutes if desired patient response is not obtained.
Clinical or Toxic Dosages of Drugs Capable of Producing the Anticholinergic Syndrome
- Case report of a 68-year-old male patient admitted to the ICU for treatment of a severe thioridazine intoxication[1].
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Physostigmine in adult patients.
Non–Guideline-Supported Use
Reversal of Postoperative Reactions to Scopolamine
- Dosage: 1.0-1.2 milligrams IV[2]. Unique dose.
Reversal of Postoperative Reactions to Scopolamine in Post-Partum
- Clinical trial of 5 postpartum patients[3].
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Clinical or Toxic Dosages of Drugs Capable of Producing the Anticholinergic Syndrome
- Case report of a 27 month-old patient with amitriptyline overdose[1].
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Physostigmine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Physostigmine in pediatric patients.
Contraindications
- Physostigmine Salicylate Injection should not be used in the presence of asthma, gangrene, diabetes, cardiovascular disease, mechanical obstruction of the intestine or urogenital tract or any vagotonic state, and in patients receiving choline esters and depolarizing neuromuscular blocking agents (decamethonium, succinylcholine).
- For post-anesthesia, the concomitant use of atropine with physostigmine salicylate is not recommended, since the atropine antagonizes the action of physostigmine.
Warnings
- Contains sodium bisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in non-asthmatic people.
- If excessive symptoms of salivation, emesis, urination and defecation occur, the use of Physostigmine Salicylate Injection should be terminated. If excessive sweating or nausea occur, the dosage should be reduced.
- Intravenous administration should be at a slow, controlled rate, no more than 1 mg per minute. Rapid administration can cause bradycardia, hypersalivation leading to a respiratory difficulties and possible convulsions. An overdosage of Physostigmine Salicylate Injection can cause a cholinergic crisis.
Adverse Reactions
Clinical Trials Experience
Cardiovascular Effects
- Asystole
- Bradyarrhythmia
- Case Report of Alzheimer patients being treated with Physostigmine[4]
- Case Report of a patient treated with physostigmine for post-operative anesthesia with Scopolamine[2].
- Cardiac dysrhythmia
- Hypertension
- Case report of a 72 year-old patient treated with 0.5 mg q2h for Alzheimer's disease[5].
- Increased cardiac output[6]
- Increased pulmonary arterial wedge pressure[6]
- Tachycardia
- Dosage: 2mg may cause tachycardia[7]
Endocrine/Metabolic Effects
- Increased leves of growth hormone
- Dosage: 12.5 mg/kg[8].
Gastrointestinal Effects
- Abnormal defecation: diarrhea
- Excessive salivation
- Hyperperistalsis
- Nausea and vomiting
Immunological Effects
Musculoeskeletal Effects
- Muscle fasciculation
- Muscle weakness
Neurological Effects
Psychiatric Depression
Renal Effects
Respiratory Effects
- Bronchospasm
- Excessive bronchial secretion[10]
Postmarketing Experience
There is limited information regarding Physostigmine Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Physostigmine Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Safe use in pregnancy and lactation has not been established; therefore, use in pregnant women, nursing mothers or women who may become pregnant requires that possible benefits be weighed against possible hazards to mother and child.
- Safe use in pregnancy and lactation has not been established; therefore, use in pregnant women, nursing mothers or women who may become pregnant requires that possible benefits be weighed against possible hazards to mother and child.
Labor and Delivery
There is no FDA guidance on use of Physostigmine during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Physostigmine in women who are nursing.
Pediatric Use
- Recommended dosage is 0.02 mg/kg; intramuscularly or by slow intravenous injection, no more than 0.5 mg per minute. If the toxic effects persist, and there is no sign of cholinergic effects, the dosage may be repeated at 5 to 10 minute intervals until a therapeutic effect is obtained or a maximum of 2 mg dosage is attained.
Geriatic Use
There is no FDA guidance on the use of Physostigmine in geriatric settings.
Gender
There is no FDA guidance on the use of Physostigmine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Physostigmine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Physostigmine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Physostigmine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Physostigmine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Physostigmine in patients who are immunocompromised.
Administration and Monitoring
Administration
- IV administration should be at allow controlled rate of no more than 1 mg per minute. Dosage may be repeated at intervals of 10 to 30 minutes if desired patient response is not obtained.
Monitoring
There is limited information regarding Physostigmine Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Physostigmine and IV administrations.
Overdosage
Can cause a cholinergic crisis. Appropriate antidote is atropine sulfate.
Pharmacology
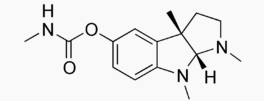
| |
Physostigmine
| |
| Systematic (IUPAC) name | |
| (3aR,8aS)-1,3a,8-Trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo[2,3-b]indol-5-yl N-methylcarbamate | |
| Identifiers | |
| CAS number | |
| ATC code | S01 V03AB19 (WHO) |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 275.346 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Major metabolite: Eseroline |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | intravenous, intramuscular, ophthalmic |
Mechanism of Action
Physostigmine Salicylate Injection is a reversible anticholinesterase which effectively increases the concentration of acetylcholine at the sites of cholinergic transmission. The action of acetylcholine is normally very transient because of its hydrolysis by the enzyme, acetylcholinesterase. Physostigmine Salicylate Injection inhibits the destructive action of acetylcholinesterase and thereby prolongs and exaggerates the effect of the acetylcholine.
Structure
There is limited information regarding Physostigmine Structure in the drug label.
Pharmacodynamics
There is limited information regarding Physostigmine Pharmacodynamics in the drug label.
Pharmacokinetics
- Physostigmine Salicylate Injection contains a tertiary amine and easily penetrates the blood brain barrier, while an anticholinesterase, such as neostigmine, which has a quaternary ammonium ion is not capable of crossing the barrier. Physostigmine Salicylate Injection can reverse both central and peripheral anticholinergia. The anticholinergic syndrome has both central and peripheral signs and symptoms. Central toxic effects include anxiety, delirium, disorientation, hallucinations, hyperactivity and seizures. Severe poisoning may produce coma, medullary paralysis and death. Peripheral toxicity is characterized by tachycardia, hyperpyrexia, mydriasis, vasodilation, urinary retention, diminution of gastrointestinal motility, decrease of secretion in salivary and sweat glands, and loss of secretions in the pharynx, bronchi, and nasal passages.
- Dramatic reversal of the effects of anticholinergic symptoms can be expected in minutes after the intravenous administration of Physostigmine Salicylate Injection, if the diagnosis is correct and the patient has not suffered anoxia or other insult. The duration of action of Physostigmine Salicylate Injection is relatively short, approximately 45 to 60 minutes.
Nonclinical Toxicology
There is limited information regarding Physostigmine Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Physostigmine Clinical Studies in the drug label.
How Supplied
- 2 mL Ampules packed 10 per box, 1 mg per mL.
Storage
- Store at 20° to 25°C (68° to 77°F)
Images
Drug Images
{{#ask: Page Name::Physostigmine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Physostigmine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Physostigmine Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Physostigmine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Physostigmine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 Schmidt W, Lang K (1997). "Life-threatening dysrhythmias in severe thioridazine poisoning treated with physostigmine and transient atrial pacing". Crit Care Med. 25 (11): 1925–30. PMID 9366781.
- ↑ 2.0 2.1 Holzgrafe RE, Vondrell JJ, Mintz SM (1973). "Reversal of postoperative reactions to scopolamine with physostigmine". Anesth Analg. 52 (6): 921–5. PMID 4796567.
- ↑ Smiler BG, Bartholomew EG, Sivak BJ, Alexander GD, Brown EM (1973). "Physostigmine reversal of scopolamine delirium in obstetric patients". Am J Obstet Gynecol. 116 (3): 326–9. PMID 4707533.
- ↑ Sano M, Bell K, Marder K, Stricks L, Stern Y, Mayeux R (1993). "Safety and efficacy of oral physostigmine in the treatment of Alzheimer disease". Clin Neuropharmacol. 16 (1): 61–9. PMID 8422658.
- ↑ Cain JW (1986). "Hypertension associated with oral administration of physostigmine in a patient with Alzheimer's disease". Am J Psychiatry. 143 (7): 910–2. PMID 3717434.
- ↑ 6.0 6.1 Nilsson E, Meretoja OA, Neuvonen P (1983). "Hemodynamic responses to physostigmine in patients with a drug overdose". Anesth Analg. 62 (10): 885–8. PMID 6614520.
- ↑ Janowsky DS, Risch SC, Kennedy B, Ziegler MG, Huey LY (1986). "Acute effects of physostigmine and neostigmine in man". Mil Med. 151 (1): 48–51. PMID 3080707.
- ↑ Mazza E, Ghigo E, Boffano G, Valetto M, Maccario M, Arvat E; et al. (1994). "Effects of direct and indirect acetylcholine receptor agonists on growth hormone secretion in humans". Eur J Pharmacol. 254 (1–2): 17–20. PMID 8206111.
- ↑ Newton RW (1975). "Physostigmine salicylate in the treatment of tricyclic antidepressant overdosage". JAMA. 231 (9): 941–3. PMID 1173100.
- ↑ Nattel S, Bayne L, Ruedy J (1979). "Physostigmine in coma due to drug overdose". Clin Pharmacol Ther. 25 (1): 96–102. PMID 363335.
{{#subobject:
|Label Page=Physostigmine |Label Name=Packagejns.png
}}