PEComa
| PEComa | |
 | |
|---|---|
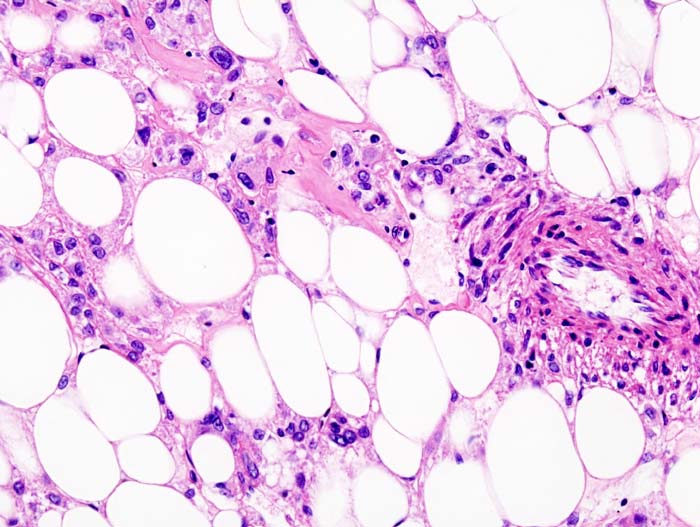
| Histopathologic image of renal angiomyolipoma. Nephrectomy specimen. H&E stain. | |
| MeSH | D054973 |
|
WikiDoc Resources for PEComa |
|
Articles |
|---|
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on PEComa at Clinical Trials.gov Clinical Trials on PEComa at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on PEComa
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating PEComa Risk calculators and risk factors for PEComa
|
|
Healthcare Provider Resources |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Zahir Ali Shaikh, MD[2]
Synonyms and keywords:Perivascular epithelioid cell tumor
Overview
The World Health Organization defines perivascular epithelioid cell tumors (PEComas) as mesenchymal tumors composed of histologically and immunohistochemically distinctive perivascular epithelioid cells (PECs). PEComas were first discovered by Pea and Colleagues in 1991. Zamboni et al in 1996 suggested the name PEComa for these neoplasms. They are a group of tumors that includes: Angiomyolipoma (AML), Clear Cell Sugar Tumor (CCST), Lymphangioleiomyomatosis (LAM) and others. PEC has no normal counterpart, it expresses myogenic and melanocytes markers such as HMB45 and actin. Genetically they are linked to the Tuberous Sclerosis Genes 1 and 2. There are no established risk factors for the PEComas but the risk increases in patients with tuberous Sclerosis. The Symptoms depend upon the area involved and can include palpable abdominal mass/abnormal vaginal bleeding (uterus), flank pain (kidney) or dull abdominal pain in the right upper quadrant (Liver). It can occur in any age group, but median age is 54 years and is more common in females. PEComas must be differentiated from Epithelioid Smooth Muscle cell tumors(epithelioid leiomyosarcoma and epithelioid leiomyoma), Malignant Melanoma, Clear cell sarcoma of tendon and aponeroses (melanoma of soft parts), Alveolar soft part sarcoma, Endometrial stromal sarcoma with clear cell features, Carcinoma (especially renal cell and adrenocortical carcinoma) and Paraganglioma. There is no evidence to recommend the routine screening. Clinically most PEComas are benign. CT scan and presence of PECs on histology are helpful in diagnosis. Surgery is the mainstay of treatment, other chemotherapeutic and immunotherapeutic drugs are under trial.
Historical Perspective
- PEComas was first discovered by Pea and Colleagues in 1991, where they first noticed this unusual cell in both Angiomyolipoma (AML) and Clear Cell Sugar Tumor of Lung (CCST).[1]
- In 1992, Bonetti and Colleagues proposed a cellular link between AML, CCST and lymphangioleiomyomatosis (LAM), their association with tuberous sclerosis complex (TSC) and advanced the concept of a family of neoplasms composed of these distinctive cells which were immunoreactive with melanocytes markers and exhibit and epitheloid appearance, a clear acidophilic cytoplasm and a perivascular distribution.[2]
- In 1996, Zamboni et al reported the first case of Pancreatic CCST and suggested the name PEComa for these neoplasms composed of a pure proliferation of Perivascular Epirhloid Cells (PECs).[3]
Classification
- The World Health Organization defines Perivascular Epithelioid Cell Tumors (PEComas) as mesenchymal tumors composed of histologically and immunohistochemically distinctive perivascular epithelioid cells (PECs).[4]
- There is no established system for the classification of PEComas because of the rarity of disease, but the PEComas are a group of tumors that includes following:[5]
- Angiomyolipoma (AML)
- Clear Cell Sugar Tumor Of Lung (CCST)
- Lymphangileiomyomatosis (LAM)
- PEComas Not Otherwise Specified (PEComas-NOS); which includes Clear Cell Myomelanocytic Tumor of falciform ligament/ligamentum teres, Abdominopelvic Sarcoma of perivascular epithelioid cells, primary extrapulmonary clear cell sugar tumor.
Pathophysiology
Perivascular Epithlioid Cell (PEC) is a cell type constantly present in a group of tumors called PEComas. It has no normal counterpart.
PEC expresses myogenic and melanocytic markers such as HMB45 and Actin.[6]
Microscopic Pathology
Perivascular Epithlioid Cells (PECs) are perivascular epithelioid cells with a clear/granular cytoplasm and round, oval, centrally located nucleus without prominent nucleoli. They have mild to any atypia.[6]
On ultrastructural analysis PEC contains microfilament bundles with electron dense condensation, numerous mitochondria and membrane bound dense granules.[7]
Genetics
The precursor cell of PEComas is currently unknown. Genetically, PECs are linked to the tuberous sclerosis genes TSC1 and TSC2.[6]
Causes
PEComas are cause by genetic factors. Mutations in the tuberous sclerosis genes TSC1 and TSC2 has been associated.[6]
Differentiating PEComa from other Diseases
- PEComas must be differentiated from:[8]
- Epithelioid Smooth Muscle cell tumors(epithelioid leiomyosarcoma and epithelioid leiomyoma)
- Malignant Melanoma
- Clear cell sarcoma of tendon and aponeroses(melanoma of soft parts)
- Alveolar soft part sarcoma
- Endometrial stromal sarcoma with clear cell features
- Carcinoma (especially renal cell and adrenocortical carcinoma)
- Paraganglioma
- Any other tumor with focal or prominent clear cell change
Epidemiology and Demographics
Patients of all age groups may develop PEComas, but the mean age at diagnosis is 54 years.[9]
Women are more commonly affected by PEComas than men.
Risk Factors
There are no established risk factors for PEComas, but the risk increases in patients with Tuberous Sclerosis as the PECs are associated with TSC1 and TSC2 genes.
Screening
There is insufficient evidence to recommend routine screening for PEComas.
Natural History, Complications, and Prognosis
- Clinically most PEComas follow a benign course.[10]
- Malignant PEComas-NOS are also reported and seen in uterus. Other sites including jejunum, prostate, pelvis, skull base, somatic soft tissue and broad ligament.
- Folpe and Colleagues suggested criteria for malignancy based of following 03 factors:[8]
Diagnosis
Diagnostic Study of Choice
There are no established criteria for the diagnosis of PEComas.
History and Symptoms
The symptoms of PEComa depend on the area involved:
- Palpable abdominal masses/abnormal vaginal bleeding (uterus).[11]
- Flank pain (kidneys).[12]
- Dull abdominal pain in right upper quadrant (liver).[13]
Physical Examination
- There are no any specific physical examination findings related to the PEComas.
Laboratory findings
Immunohistochemical markers
PECs typically stain for melanocytic markers (HMB-45, HMSA-1, Melan A (Mart 1), microophthalmia transcription factor (Mitf)), myogenic markers (actin)and less commonly desmin. Immunoreactivity for vimentin is unclear.[6]
Electrocardiogram
There are no ECG findings associated with PEComas.
X-ray
There are no x-ray findings associated with PEDComas.
Echocardiography or Ultrasound
There are no echocardiography/ultrasound findings associated with PEComas.
CT scan
CT scan may be helpful in the diagnosis of PEComas. Findings on CT scan suggestive of PEComas include:
- Hypodense (15-50 hours field units) partially solid and partially cystic tumor with moderate contrast uptake (in kidney).[12]
- Hypervascular tumor with delayed washout pattern in portal venous phase (in liver).[13]
- Mass with no other pathology (in uterus). [11]
MRI
There are no MRI findings associated with PEComas.
Other Imaging Findings
There are no other imaging findings associated with PEComas.
Other Diagnostic Studies
There are no other diagnostic studies associated with PEComas.
Treatment
Medical Therapy
- There is no established medical therapy for PEComas.
- Following chemotherapeutic and immunotherapeutic drugs have been tried with mixed results: [14][15][16]
Surgery
Surgery is the mainstay of treatment for PEComas, as well as for local recurrences and metastasis, with the aim of obtaining clear resection margins.[17]
Primary Prevention
There are no established measures for the primary prevention of PEComas.
Secondary Prevention
There are no established measures for the secondary prevention of PEComas.
References
- ↑ Pea M, Bonetti F, Zamboni G, Martignoni G, Fiore-Donati L, Doglioni C (1991). "Clear cell tumor and angiomyolipoma". Am J Surg Pathol. 15 (2): 199–202. PMID 2025321.
- ↑ Bonetti F, Pea M, Martignoni G, Zamboni G (1992). "PEC and sugar". Am J Surg Pathol. 16 (3): 307–8. PMID https://www.ncbi.nlm.nih.gov/pubmed/1599021 Check
|pmid=value (help). - ↑ Zamboni G, Pea M, Martignoni G, Zancanaro C, Faccioli G, Gilioli E; et al. (1996). "Clear cell "sugar" tumor of the pancreas. A novel member of the family of lesions characterized by the presence of perivascular epithelioid cells". Am J Surg Pathol. 20 (6): 722–30. PMID https://www.ncbi.nlm.nih.gov/pubmed/8651352 Check
|pmid=value (help). - ↑ Fletcher, Christopher (2002). Pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press. ISBN 9789283224136.
- ↑ Fletcher, Christopher (2013). WHO classification of tumours of soft tissue and bone. Lyon: IARC Press. ISBN 978-9283224341.
- ↑ 6.0 6.1 6.2 6.3 6.4 Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F (2008). "PEComas: the past, the present and the future". Virchows Arch. 452 (2): 119–32. doi:10.1007/s00428-007-0509-1. PMC 2234444. PMID https://www.ncbi.nlm.nih.gov/pubmed/18080139 Check
|pmid=value (help). - ↑ D. A. Weeks, R. L. Malott, M. Arnesen, C. Zuppan, D. Aitken & G. Mierau (1991). "Hepatic angiomyolipoma with striated granules and positivity with melanoma--specific antibody (HMB-45): a report of two cases". Ultrastructural pathology. 15 (4–5): 563–571. PMID 1755113. Unknown parameter
|month=ignored (help) - ↑ 8.0 8.1 Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW (2005). "Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature". Am J Surg Pathol. 29 (12): 1558–75. PMID https://www.ncbi.nlm.nih.gov/pubmed/16327428 Check
|pmid=value (help). - ↑ Vang R, Kempson RL (2002). "Perivascular epithelioid cell tumor ('PEComa') of the uterus: a subset of HMB-45-positive epithelioid mesenchymal neoplasms with an uncertain relationship to pure smooth muscle tumors". Am J Surg Pathol. 26 (1): 1–13. PMID https://www.ncbi.nlm.nih.gov/pubmed/11756764 Check
|pmid=value (help). - ↑ Fletcher, Christopher (2013). WHO classification of tumours of soft tissue and bone. Lyon: IARC Press. ISBN 978-9283224341.
- ↑ 11.0 11.1 Theofanakis C, Thomakos N, Sotiropoulou M, Rodolakis A (2016). "Perivascular epithelioid cell tumor of the uterus: Report of two cases and mini-review of the literature". Int J Surg Case Rep. 28: 85–87. doi:10.1016/j.ijscr.2016.09.017. PMC 5043388. PMID https://www.ncbi.nlm.nih.gov/pubmed/27689526 Check
|pmid=value (help). - ↑ 12.0 12.1 D'Andrea D, Hanspeter E, D'Elia C, Martini T, Pycha A (2016). "Malignant Perivascular Epithelioid Cell Neoplasm (PEComa) of the Pelvis: A Case Report". Urol Case Rep. 6: 36–8. doi:10.1016/j.eucr.2016.02.004. PMC 4855909. PMID https://www.ncbi.nlm.nih.gov/pubmed/27169023 Check
|pmid=value (help). - ↑ 13.0 13.1 Cheung TT, Trendell-Smith N, Poon RT (2013). "Primary perivascular epithelioid cell tumour (PEComa) of the liver". BMJ Case Rep. 2013. doi:10.1136/bcr-2013-008706. PMC 3736252. PMID https://www.ncbi.nlm.nih.gov/pubmed/23845671 Check
|pmid=value (help). - ↑ Rigby, Heather; Yu, Weiming; Schmidt, Matthias H.; Fernandez, Conrad V. (2005). "Lack of response of a metastatic renal perivascular epithelial cell tumor (PEComa) to successive courses of DTIC based-therapy and imatinib mesylate". Pediatric Blood & Cancer. 45 (2): 202–206. doi:10.1002/pbc.20305. ISSN 1545-5009.
- ↑ Parfitt, Jeremy R; Bella, Anthony J; Wehrli, Bret M; Izawa, Jonathan I (2006). "Primary PEComa of the bladder treated with primary excision and adjuvant interferon-alpha immunotherapy: a case report". BMC Urology. 6 (1). doi:10.1186/1471-2490-6-20. ISSN 1471-2490.
- ↑ In-sang Jeon & Sung Moon Lee (2005). "Multimodal treatment using surgery, radiotherapy, and chemotherapy in a patient with a perivascular epithelioid cell tumor of the uterus". Journal of pediatric hematology/oncology. 27 (12): 681–684. PMID 16344678. Unknown parameter
|month=ignored (help) - ↑ Dimmler, A (2003). "Late pulmonary metastasis in uterine PEComa". Journal of Clinical Pathology. 56 (8): 627–628. doi:10.1136/jcp.56.8.627. ISSN 0021-9746.