Neuromedin B: Difference between revisions
m (Robot: Automated text replacement (-{{SIB}} +, -{{EH}} +, -{{EJ}} +, -{{Editor Help}} +, -{{Editor Join}} +)) |
Matt Pijoan (talk | contribs) m (1 revision imported) |
||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{protein | {{infobox protein | ||
|Name=neuromedin B | |Name=neuromedin B | ||
|caption= | |caption= | ||
| Line 18: | Line 18: | ||
|LocusSupplementaryData=-qter | |LocusSupplementaryData=-qter | ||
}} | }} | ||
{{ | '''Neuromedin B''' (NMB) is a [[bombesin]]-related [[peptide]] in mammals.<ref name="pmid10840151">{{cite journal | author = Ohki-Hamazaki H | title = Neuromedin B | journal = Prog. Neurobiol. | volume = 62 | issue = 3 | pages = 297–312 |date=October 2000 | pmid = 10840151 | doi = 10.1016/S0301-0082(00)00004-6 | url = }}</ref><ref name="pmid18055507">{{cite journal |vauthors=Jensen RT, Battey JF, Spindel ER, Benya RV | title = International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states | journal = Pharmacol. Rev. | volume = 60 | issue = 1 | pages = 1–42 |date=March 2008 | pmid = 18055507 | doi = 10.1124/pr.107.07108 | url = | pmc = 2517428 }}</ref> It was originally purified from pig spinal cord, and later shown to be present in human [[central nervous system]] and [[gastrointestinal tract]].<ref name="pmid2458345">{{cite journal |vauthors=Krane IM, Naylor SL, Helin-Davis D, Chin WW, Spindel ER | title = Molecular cloning of cDNAs encoding the human bombesin-like peptide neuromedin B. Chromosomal localization and comparison to cDNAs encoding its amphibian homolog ranatensin | journal = J. Biol. Chem. | volume = 263 | issue = 26 | pages = 13317–23 | date=15 September 1988| pmid = 2458345 | url = http://www.jbc.org/cgi/content/abstract/263/26/13317 | issn = }}</ref> | ||
== Sequence == | |||
The sequence of the C-terminal decapeptide is highly conserved across mammalian species: GNLWATGHFM-(NH2); this decapeptide is sometimes noted as neuromedin B, but it is more accurately described as neuromedin B 23-32. The sequence of neuromedin B (in rat) is : TPFSWDLPEPRSRASKIRVHPRGNLWATGHFM-(NH2).<ref name="pmid2398368">{{cite journal |vauthors=Wada E, Way J, Lebacq-Verheyden AM, Battey JF | title = Neuromedin B and gastrin-releasing peptide mRNAs are differentially distributed in the rat nervous system | journal = J. Neurosci. | volume = 10 | issue = 9 | pages = 2917–30 | date=1 September 1990| pmid = 2398368 | url = http://www.jneurosci.org/cgi/content/abstract/10/9/2917 | issn = }}</ref> | |||
''' | == Function == | ||
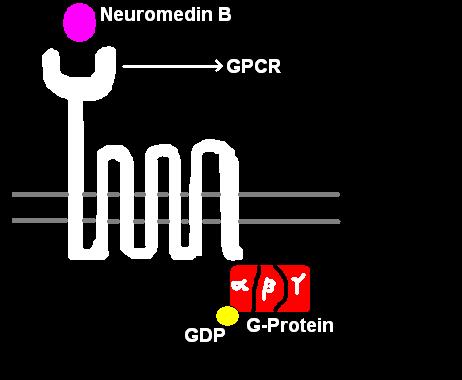
[[Image:Gpcr2.JPG|thumb|'''Figure 1''': NMB, 7-TMR receptor and G-protein]] | |||
[[Image:Gpcr+cycle.JPG|thumb|'''Figure 2''' : Signal Cascade after NMB binding]] | |||
Neuromedin regulates the following functions: | |||
* [[exocrine gland|exocrine]] and [[endocrine system|endocrine]] secretions | |||
* cell growth | |||
* body temperature | |||
* blood pressure and glucose level | |||
Neuromedin | == Neuromedin signaling pathway == | ||
NMB acts by binding to its high affinity cell surface receptor, [[neuromedin B receptor]] (NMBR). This receptor is a [[G protein-coupled receptor]] with seven transmembrane spanning regions, hence the receptor is also denoted as a 7-transmembrane receptor (7-TMR). Upon binding several intracellular signaling pathways are triggered (see '''Figure 2'''). | |||
[[ | When NMB binds to its 7-TMR, the [[heterotrimeric G protein]] that is attached to the receptor is activated. The G-protein is called heterotrimeric because it consists of 3 polypeptides: α subunit, β subunit, and γ subunit. In the activated NMBR/G-protein complex, there occurs an exchange of [[Guanosine triphosphate|GTP]] for [[Guanosine diphosphate|GDP]] bound to G-α subunit. The G-α subunit, in turn, dissociated form the G-βγ subunits. The free G-α inactivates [[adenylate cyclase]] (AC), which, in turn, catalyzes the conversion of ATP to cAMP, the latter of which functioning as a [[second messenger]]. cAMP activates of the enzyme [[Protein Kinase A]] (PKA). PKA enters the nucleus and activates the [[CREB|cAMP response element-binding]] protein. The activated CREB binds along with [[CREB binding protein]], co-activator to the CRE region of the DNA in the nucleus. CREB and CBP are held together by [[leucine zipper]]s. CRE is the control that activates number of growth factors, and thus cell proliferation and some anti-apoptotic genes. In the brain, CREB plays a role in long-term memory and learning. | ||
[[ | {{Clear}} | ||
== | ==References== | ||
{{Reflist|2}} | |||
==External links== | |||
* {{MeshName|Neuromedin+B}} | |||
{{Neuropeptides}} | |||
[[Category:Neuropeptides]] | [[Category:Neuropeptides]] | ||
Latest revision as of 12:37, 9 January 2019
| neuromedin B | |
|---|---|
| Identifiers | |
| Symbol | NMB |
| Entrez | 4828 |
| HUGO | 7842 |
| OMIM | 162340 |
| RefSeq | NM_021077 |
| UniProt | P08949 |
| Other data | |
| Locus | Chr. 15 q11-qter |
Neuromedin B (NMB) is a bombesin-related peptide in mammals.[1][2] It was originally purified from pig spinal cord, and later shown to be present in human central nervous system and gastrointestinal tract.[3]
Sequence
The sequence of the C-terminal decapeptide is highly conserved across mammalian species: GNLWATGHFM-(NH2); this decapeptide is sometimes noted as neuromedin B, but it is more accurately described as neuromedin B 23-32. The sequence of neuromedin B (in rat) is : TPFSWDLPEPRSRASKIRVHPRGNLWATGHFM-(NH2).[4]
Function

Neuromedin regulates the following functions:
Neuromedin signaling pathway
NMB acts by binding to its high affinity cell surface receptor, neuromedin B receptor (NMBR). This receptor is a G protein-coupled receptor with seven transmembrane spanning regions, hence the receptor is also denoted as a 7-transmembrane receptor (7-TMR). Upon binding several intracellular signaling pathways are triggered (see Figure 2).
When NMB binds to its 7-TMR, the heterotrimeric G protein that is attached to the receptor is activated. The G-protein is called heterotrimeric because it consists of 3 polypeptides: α subunit, β subunit, and γ subunit. In the activated NMBR/G-protein complex, there occurs an exchange of GTP for GDP bound to G-α subunit. The G-α subunit, in turn, dissociated form the G-βγ subunits. The free G-α inactivates adenylate cyclase (AC), which, in turn, catalyzes the conversion of ATP to cAMP, the latter of which functioning as a second messenger. cAMP activates of the enzyme Protein Kinase A (PKA). PKA enters the nucleus and activates the cAMP response element-binding protein. The activated CREB binds along with CREB binding protein, co-activator to the CRE region of the DNA in the nucleus. CREB and CBP are held together by leucine zippers. CRE is the control that activates number of growth factors, and thus cell proliferation and some anti-apoptotic genes. In the brain, CREB plays a role in long-term memory and learning.
References
- ↑ Ohki-Hamazaki H (October 2000). "Neuromedin B". Prog. Neurobiol. 62 (3): 297–312. doi:10.1016/S0301-0082(00)00004-6. PMID 10840151.
- ↑ Jensen RT, Battey JF, Spindel ER, Benya RV (March 2008). "International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states". Pharmacol. Rev. 60 (1): 1–42. doi:10.1124/pr.107.07108. PMC 2517428. PMID 18055507.
- ↑ Krane IM, Naylor SL, Helin-Davis D, Chin WW, Spindel ER (15 September 1988). "Molecular cloning of cDNAs encoding the human bombesin-like peptide neuromedin B. Chromosomal localization and comparison to cDNAs encoding its amphibian homolog ranatensin". J. Biol. Chem. 263 (26): 13317–23. PMID 2458345.
- ↑ Wada E, Way J, Lebacq-Verheyden AM, Battey JF (1 September 1990). "Neuromedin B and gastrin-releasing peptide mRNAs are differentially distributed in the rat nervous system". J. Neurosci. 10 (9): 2917–30. PMID 2398368.
External links
- Neuromedin+B at the US National Library of Medicine Medical Subject Headings (MeSH)