Nesiritide: Difference between revisions
No edit summary |
No edit summary |
||
| Line 106: | Line 106: | ||
|contraindications= | |contraindications= | ||
* | * Persistent [[SBP|systolic blood pressure]] <100 mm Hg prior to therapy because of an increased risk of symptomatic [[hypotension]] | ||
* [[Hypersensitivity]] to any of its components | |||
* [[Cardiogenic shock]] | |||
<!--Warnings--> | <!--Warnings--> | ||
Revision as of 04:32, 2 July 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Nesiritide is a natriuretic peptide that is FDA approved for the {{{indicationType}}} of patients with acutely decompensated heart failure who have dyspnea at rest or with minimal activity. Common adverse reactions include hypotension, nausea, dizziness, headache, and elevated serum creatinine.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Acute Decompensated Heart Failure
- NATRECOR® (nesiritide) is for intravenous (IV) use only. There is limited experience with administering NATRECOR® for longer than 96 hours. Monitor blood pressure closely during NATRECOR® administration.
- Dosing Information
- Recommended Dosage: IV bolus of 2 μg/kg followed by a continuous infusion of 0.01 μg/kg/min. Do not initiate NATRECOR® at a dose that is above the recommended dose.
- The loading dose may not be appropriate for those with low systolic blood pressure (SBP) <110 mm Hg or for patients recently treated with afterload reducers.
- The administration of the recommended dose of NATRECOR® is a two step process:
- Step 1. Administration of the IV Bolus
- After preparation of the infusion bag, withdraw the bolus volume (see Table 1) from the NATRECOR® infusion bag, and administer it over approximately 60 seconds through an IV port in the tubing.

- Bolus Volume (mL) = Patient Weight (kg) / 3
- Step 2. Administration of the Continuous Infusion
- Immediately following the administration of the bolus, infuse NATRECOR® at a flow rate of 0.1 mL/kg/hr. This will deliver a NATRECOR® infusion dose of 0.01 mcg/kg/min.
- To calculate the infusion flow rate to deliver a 0.01 mcg/kg/min dose, use the following formula (see Table 2):
- Infusion Flow Rate (mL/hr) = Patient Weight (kg) × 0.1
- Step 2. Administration of the Continuous Infusion

Dose Adjustments
- The dose-limiting side effect of NATRECOR® is hypotension. If hypotension occurs during the administration of NATRECOR®, reduce the dose of or discontinue NATRECOR® and initiate other measures to support blood pressure (IV fluids, changes in body position). When symptomatic hypotension occurs, discontinue NATRECOR®. Because hypotension caused by NATRECOR® may be prolonged (up to hours), a period of observation may be necessary before restarting the drug. NATRECOR® may be subsequently restarted at a dose that is reduced by 30% (with no bolus administration) once the patient has stabilized.
- Do not up-titrate NATRECOR® more frequently than every 3 hours. Use central hemodynamic monitoring and do not exceed 0.03 mcg/kg/min.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nesiritide in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nesiritide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
The safety and effectiveness of NATRECOR® in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nesiritide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nesiritide in pediatric patients.
Contraindications
- Persistent systolic blood pressure <100 mm Hg prior to therapy because of an increased risk of symptomatic hypotension
- Hypersensitivity to any of its components
- Cardiogenic shock
Warnings
Description
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Nesiritide in the drug label.
Central Nervous System
Cardiovascular
Respiratory
Gastrointestinal
Hypersensitivity
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Nesiritide in the drug label.
Central Nervous System
Cardiovascular
Respiratory
Gastrointestinal
Hypersensitivity
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Nesiritide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Nesiritide during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Nesiritide with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Nesiritide with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Nesiritide with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Nesiritide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Nesiritide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Nesiritide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Nesiritide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Nesiritide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Nesiritide in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
Preparation and Administration Instructions
- The NATRECOR® bolus must be drawn from the prepared infusion bag. Prime the IV tubing with 5 mL of the solution for infusion prior to connecting to the patient's vascular access port and prior to administering the bolus or starting the infusion.
- Reconstitute one 1.5 mg vial of NATRECOR® by adding 5 mL of diluent removed from a pre-filled 250 mL plastic IV bag containing the diluent of choice. After reconstitution of the vial, each mL contains 0.32 mg of nesiritide. The following preservative-free diluents are recommended for reconstitution: 5% Dextrose Injection (D5W), USP; 0.9% Sodium Chloride Injection, USP; 5% Dextrose and 0.45% Sodium Chloride Injection, USP, or 5% Dextrose and 0.2% Sodium Chloride Injection, USP.
- Do not shake the vial. Rock the vial gently so that all surfaces, including the stopper, are in contact with the diluent to ensure complete reconstitution. Use only a clear, essentially colorless solution.
- Withdraw the entire contents of the reconstituted NATRECOR® vial and add to the 250 mL plastic IV bag. This will yield a solution with a concentration of NATRECOR® of approximately 6 mcg/mL. Invert the IV bag several times to ensure complete mixing of the solution.
- Use the reconstituted solution within 24 hours, as NATRECOR® contains no antimicrobial preservative. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Reconstituted vials of NATRECOR® may be stored at 2 to 25°C (36 to 77°F) for up to 24 hours.
IV Compatibility
There is limited information regarding IV Compatibility of Nesiritide in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
Description
Management
Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Nesiritide in the drug label.
Pharmacology
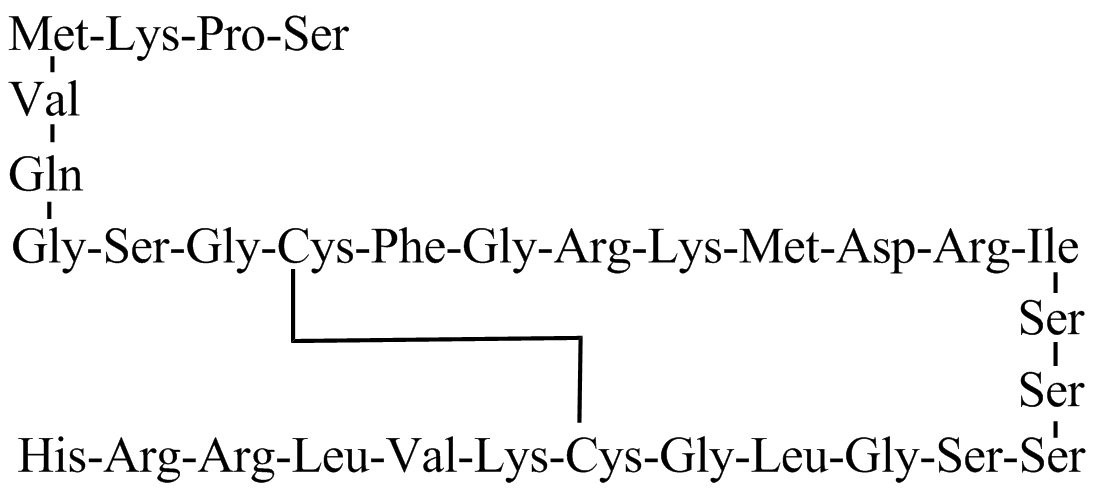
| |
Nesiritide
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | |
| ATC code | C01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 3464 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | IV only |
Mechanism of Action
There is limited information regarding Nesiritide Mechanism of Action in the drug label.
Structure
There is limited information regarding Structure of Nesiritide in the drug label.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Nesiritide in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Nesiritide in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Nesiritide in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Nesiritide in the drug label.
Condition1
Description
How Supplied
There is limited information regarding Nesiritide How Supplied in the drug label.
Storage
There is limited information regarding Nesiritide Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Nesiritide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Nesiritide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Nesiritide in the drug label.
Precautions with Alcohol
Alcohol-Nesiritide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Natrecor®
Look-Alike Drug Names
- Natrecor® — Norcuron®[1]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Nesiritide |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Nesiritide |Label Name=No image.jpg
}}