Myxoma pathophysiology: Difference between revisions
No edit summary |
No edit summary |
||
| Line 19: | Line 19: | ||
[http://www.peir.net Images shown below are courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology] | [http://www.peir.net Images shown below are courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology] | ||
<div align=" | <div align="center"> | ||
<gallery heights="225" widths="225"> | <gallery heights="225" widths="225"> | ||
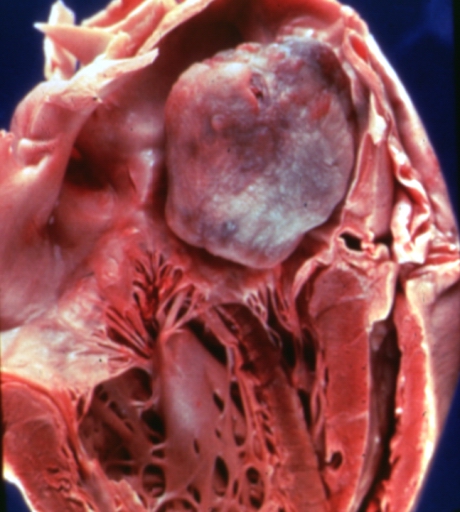
Image:Atrial myxoma 1.jpg|A gelatinous tumor is attached by a narrow pedicle to the atrial septum. The myxoma has an irregular surface and nearly fills the left atrium. | Image:Atrial myxoma 1.jpg|A gelatinous tumor is attached by a narrow pedicle to the atrial septum. The myxoma has an irregular surface and nearly fills the left atrium. | ||
Image:Left atrial myxoma 1.jpg|Left Atrial Myxoma | Image:Left atrial myxoma 1.jpg|Left Atrial Myxoma | ||
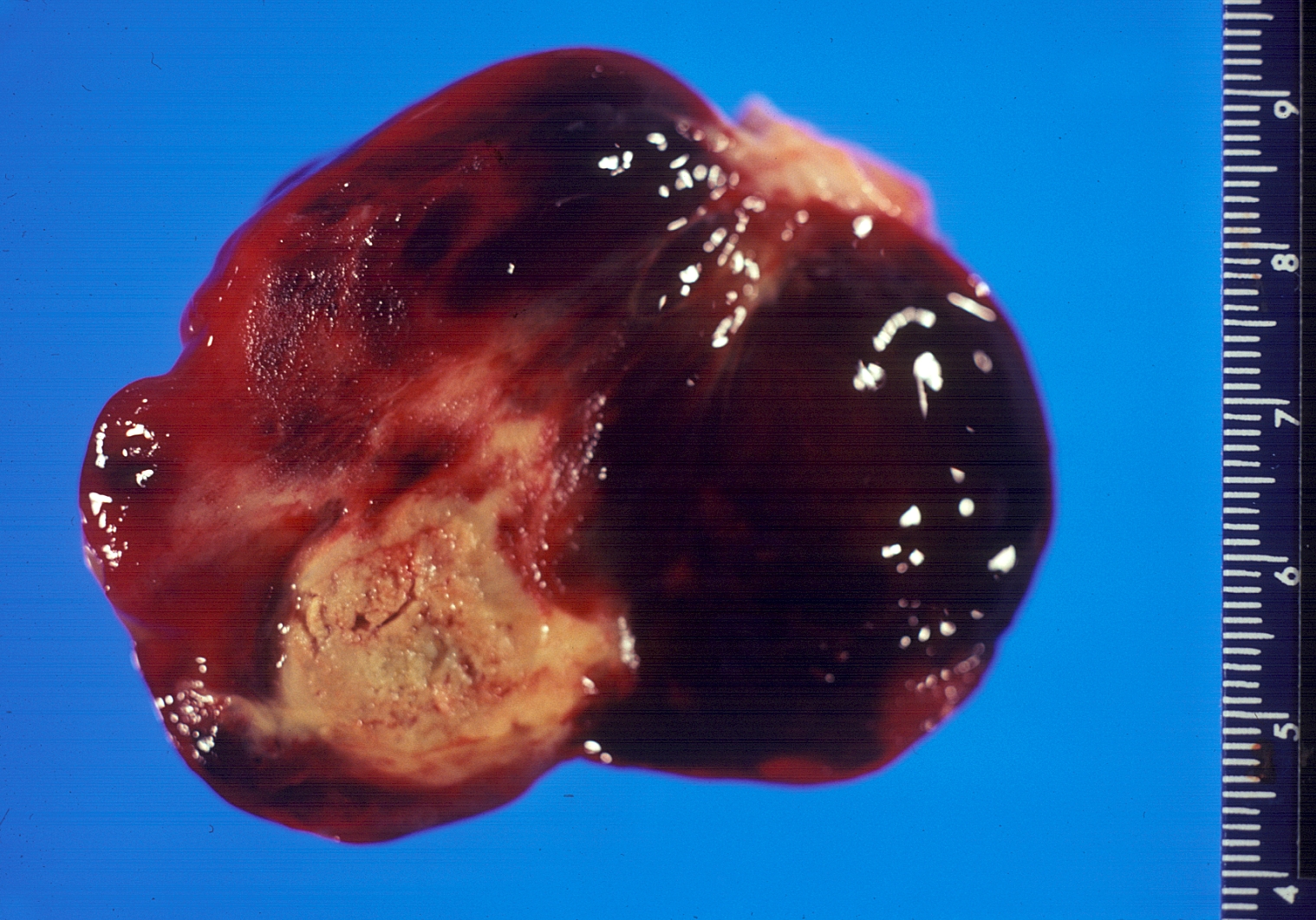
Image:Gross myxoma.jpg|Gross pathology Atrial Myxoma | |||
</gallery> | </gallery> | ||
</div> | </div> | ||
<div align="left"> | <div align="left"> | ||
<gallery heights="225" widths="225"> | <gallery heights="225" widths="225"> | ||
</gallery> | </gallery> | ||
</div> | </div> | ||
<div align="left"> | <div align="left"> | ||
<gallery heights="225" widths="225"> | <gallery heights="225" widths="225"> | ||
| Line 44: | Line 43: | ||
It is also common to find hemosiderin within the histiocytes. Thrombosis, fibrosis and calcification are a frequent histological finding. In some cases, extramedular hematopoises is present and mucin-producing glands can be also seen in the base of the tumor. | It is also common to find hemosiderin within the histiocytes. Thrombosis, fibrosis and calcification are a frequent histological finding. In some cases, extramedular hematopoises is present and mucin-producing glands can be also seen in the base of the tumor. | ||
<div align="center"> | |||
<div align=" | |||
<gallery heights="225" widths="225"> | <gallery heights="225" widths="225"> | ||
Image: | Image:800px-Atrial myxoma edge high mag.jpg|1) Black arrow(top):Endothelium 2) Black arrow(bottom): Hemosiderin macrophage. <ref> Cardiac Myxoma. Libre Pathology URL http://librepathology.org/wiki/index.php/Cardiac_myxoma Accessed on November 19,2015 </ref> | ||
</gallery> | </gallery> | ||
</div> | </div> | ||
<div align=" | |||
<gallery heights=" | [http://www.peir.net Images shown below are courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology] | ||
<div align="center"> | |||
<gallery heights="150" widths="150"> | |||
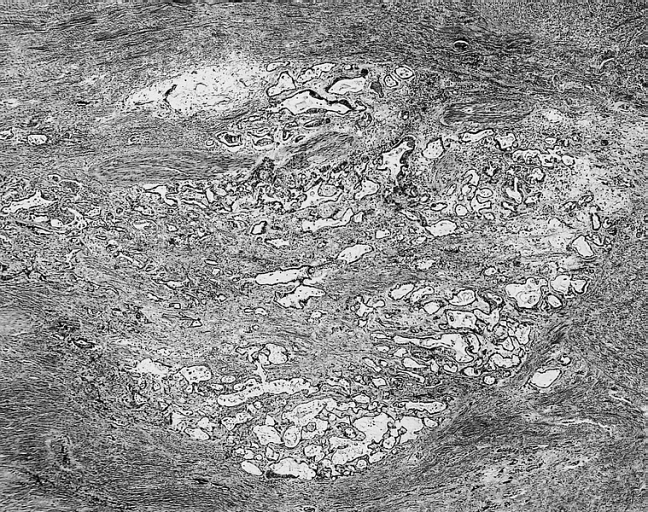
Image:Cardiac myxoma mic 2.jpg|Cardiac Myxoma: Gamna Bodies: A peculiar form of fibrosis with deposition of iron pigment, identical to that seen in the spleens of patients with sickle cell anemia, is not uncommon in myxoma. | |||
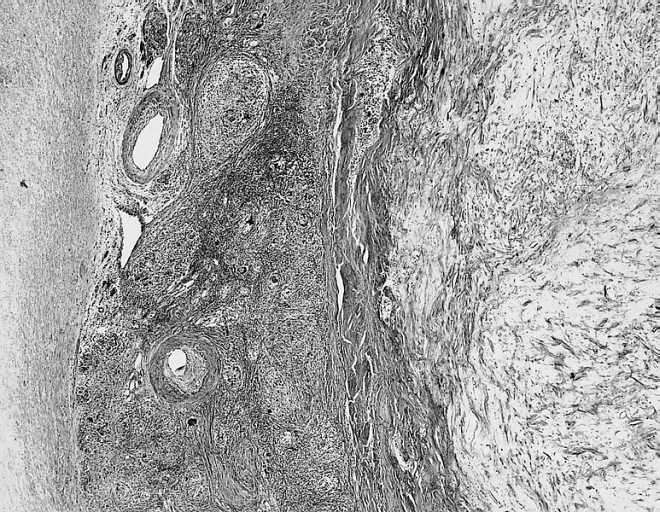
Image:Cardiac myxoma mic 3.jpg|Cardiac Myxoma Common features at the interface with the atrial septum include lymphoid aggregates, smooth muscle bundles, and thick walled vessels which angiographically may look like neovascularization. | Image:Cardiac myxoma mic 3.jpg|Cardiac Myxoma Common features at the interface with the atrial septum include lymphoid aggregates, smooth muscle bundles, and thick walled vessels which angiographically may look like neovascularization. | ||
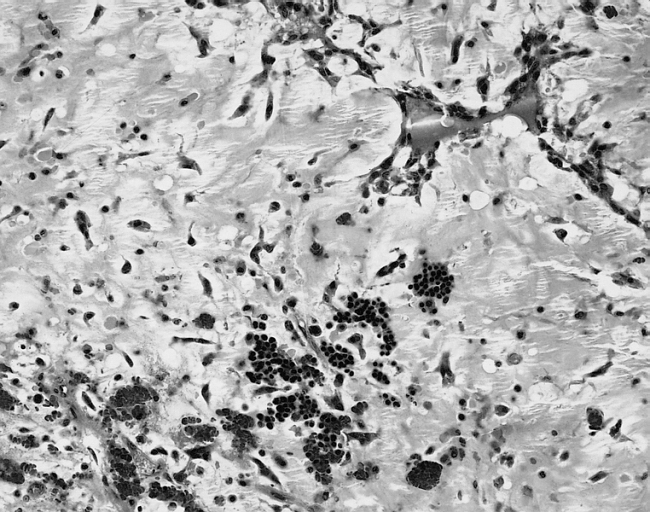
Image:Cardiac myxoma mic 4.jpg|Cardiac Myxoma The extramedullary hematopoiesis seen here is present in about 7 percent of cardiac myxomas. | Image:Cardiac myxoma mic 4.jpg|Cardiac Myxoma The extramedullary hematopoiesis seen here is present in about 7 percent of cardiac myxomas. | ||
Image:Cardiac myxoma mic 5.jpg|Cardiac Myxoma Glandular structures are seen in less than 5 percent of cases. In this example, they were limited to the base of the myxoma | Image:Cardiac myxoma mic 5.jpg|Cardiac Myxoma Glandular structures are seen in less than 5 percent of cases. In this example, they were limited to the base of the myxoma | ||
</gallery> | </gallery> | ||
| Line 66: | Line 62: | ||
==Immunohistochemistry== | ==Immunohistochemistry== | ||
Cardiac myxoma cells exhibit immuno-reactivity mainly for calretinin (75–100%) followed by vimentin (>50%), Notch-1, alpha-1 antichymotrypsin and plakophilin- 2.<ref name="pmid11642722">{{cite journal |vauthors=Acebo E, Val-Bernal JF, Gómez-Roman JJ |title=Thrombomodulin, calretinin and c-kit (CD117) expression in cardiac myxoma |journal=Histol. Histopathol. |volume=16 |issue=4 |pages=1031–6 |year=2001 |pmid=11642722 |doi= |url=}}</ref>Calretinin plays an important role in the discrimination of mural thrombi and papillary fibroelastoma.<ref name="pmid11642722">{{cite journal |vauthors=Acebo E, Val-Bernal JF, Gómez-Roman JJ |title=Thrombomodulin, calretinin and c-kit (CD117) expression in cardiac myxoma |journal=Histol. Histopathol. |volume=16 |issue=4 |pages=1031–6 |year=2001 |pmid=11642722 |doi= |url=}}</ref>. Another immunohistochemical marker, survivin (an apoptosis inhibitor) has been detected to play an important role in the development and growth of cardiac myxomas.<ref name="pmid21880190">{{cite journal |vauthors=Lin YS, Jung SM, Wu HH, Shiu TF, Tzai FC, Chu JJ, Lin PJ, Chu PH |title=Survivin expression in cardiac myxoma |journal=Chang Gung Med J |volume=34 |issue=4 |pages=360–6 |year=2011 |pmid=21880190 |doi= |url=}}</ref> | Cardiac myxoma cells exhibit immuno-reactivity mainly for calretinin (75–100%) followed by vimentin (>50%), Notch-1, alpha-1 antichymotrypsin and plakophilin- 2.<ref name="pmid11642722">{{cite journal |vauthors=Acebo E, Val-Bernal JF, Gómez-Roman JJ |title=Thrombomodulin, calretinin and c-kit (CD117) expression in cardiac myxoma |journal=Histol. Histopathol. |volume=16 |issue=4 |pages=1031–6 |year=2001 |pmid=11642722 |doi= |url=}}</ref>Calretinin plays an important role in the discrimination of mural thrombi and papillary fibroelastoma.<ref name="pmid11642722">{{cite journal |vauthors=Acebo E, Val-Bernal JF, Gómez-Roman JJ |title=Thrombomodulin, calretinin and c-kit (CD117) expression in cardiac myxoma |journal=Histol. Histopathol. |volume=16 |issue=4 |pages=1031–6 |year=2001 |pmid=11642722 |doi= |url=}}</ref>. Another immunohistochemical marker, survivin (an apoptosis inhibitor) has been detected to play an important role in the development and growth of cardiac myxomas.<ref name="pmid21880190">{{cite journal |vauthors=Lin YS, Jung SM, Wu HH, Shiu TF, Tzai FC, Chu JJ, Lin PJ, Chu PH |title=Survivin expression in cardiac myxoma |journal=Chang Gung Med J |volume=34 |issue=4 |pages=360–6 |year=2011 |pmid=21880190 |doi= |url=}}</ref> | ||
==Genetics== | ==Genetics== | ||
Single cardiac myxomas and familial forms are related with several chromosome and gene alterations which involve cardiac development. | Single cardiac myxomas and familial forms are related with several chromosome and gene alterations which involve cardiac development. | ||
Inherited myxomas are usually presented in the [[Carney complex]]. The development of this syndrome is a result of [[PRKAR1A]] gene inactivation mutation that is associated with [[chromosome]] 17q24.2-q24.3. This gene plays an important role in cardiac development and myxomagenesis. The expression of PRKAR1A causes myxomatous changes in the endocardium.<ref name="pmid26416542">{{cite journal |vauthors=Sun Y, Chen X, Sun J, Wen X, Liu X, Zhang Y, Hoffman AR, Hu JF, Gao Y |title=A Novel Inherited Mutation in PRKAR1A Abrogates PreRNA Splicing in a Carney Complex Family |journal=Can J Cardiol |volume=31 |issue=11 |pages=1393–401 |year=2015 |pmid=26416542 |doi=10.1016/j.cjca.2015.05.018 |url=}}</ref> | Inherited myxomas are usually presented in the [[Carney complex]]. The development of this syndrome is a result of [[PRKAR1A]] gene inactivation mutation that is associated with [[chromosome]] 17q24.2-q24.3. This gene plays an important role in cardiac development and myxomagenesis. The expression of PRKAR1A causes myxomatous changes in the endocardium.<ref name="pmid26416542">{{cite journal |vauthors=Sun Y, Chen X, Sun J, Wen X, Liu X, Zhang Y, Hoffman AR, Hu JF, Gao Y |title=A Novel Inherited Mutation in PRKAR1A Abrogates PreRNA Splicing in a Carney Complex Family |journal=Can J Cardiol |volume=31 |issue=11 |pages=1393–401 |year=2015 |pmid=26416542 |doi=10.1016/j.cjca.2015.05.018 |url=}}</ref> | ||
Revision as of 18:56, 19 November 2015
|
Myxoma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Myxoma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Myxoma pathophysiology |
|
Risk calculators and risk factors for Myxoma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Maria Fernanda Villarreal, M.D. [2]Cafer Zorkun, M.D., Ph.D. [3]Ahmad Al Maradni, M.D. [4]
Overview
Cardiac myxoma contains undifferentiated mesenchymal cells, which potentially differentiate into many tissues such as blood vessels, glandular structures, bones, and source of extramedullary hematopoiesis.[1]
Pathophysiology
Cardiac myxoma arises from remnants of subendocardial vasoformative reserve cells, which are primitive mesenchymal cells that are normally involved in the supportive structure of the endocardium. [2] [3] Myxomas are usually located in the fossa ovalis and endocardium of the atrial septum.
Gross Pathology
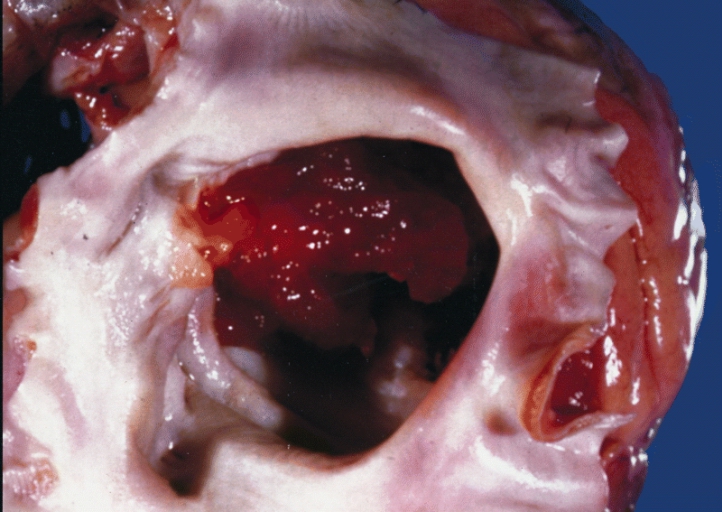
On gross pathology, external appearance, consistency size and weight are extremely variable findings of cardiac myxoma. Tumor consistency depends on the quantity and distribution of fibrous tissue and calcification (It can be smooth, lobulated, friable or gelatinous). [4] Usually a macroscopic gelatinous, irregular surface that fills the left atrium is a characteristic finding of myxoma. Myxomas that have irregular consistency are more likely to form surface thrombi and embolize.
Morphologically, these lesions tend to be attached to the endocardium by a broad-based pedunculated stalk. In some cases, the attachment to the endocardium can also be without a clear stalk, or sessile. Cardiac myxomas are non-invasive tumors, thus there is no infiltration to underlying tissues.
Cardiac myxomas are intracavitary tumors. The distribution is normally within the interatrial septum or adjacent to foramen ovale (75%). However, they can also be found in other cardiac chambers, such as right atrium (15%),ventricles(˜2%)or cardiac valves (rare).[5] Large cardiac myxomas are usually located in fossa ovalis. The average tumor size is from 0.6 to 12 cm, with a mean weight of 40 g.[6]
-
A gelatinous tumor is attached by a narrow pedicle to the atrial septum. The myxoma has an irregular surface and nearly fills the left atrium.
-
Left Atrial Myxoma
-
Gross pathology Atrial Myxoma
Microscopic Pathology
On microscopic histopathological analysis, myxoma cells have an ovoid nucleus with large nucleoli, abundant eosinophilic cytoplasm, and indistinct cell borders. They are usually arranged in perivascular ring structures (typically, infiltrated by lymphocytes and macrophages). The Gamna-Bodies consist of fibrosis and deposition of iron pigments are a characteristic finding of myxoma tumors.
The extracellular matrix forms an Alcian blue-positive myxoid stroma, composed of variable amounts of proteoglycans, elastin and collagen. There is a diffuse reticulated stroma with fine collagen fibrils on which iron encrustation often occurs. [4]
It is also common to find hemosiderin within the histiocytes. Thrombosis, fibrosis and calcification are a frequent histological finding. In some cases, extramedular hematopoises is present and mucin-producing glands can be also seen in the base of the tumor.
-
1) Black arrow(top):Endothelium 2) Black arrow(bottom): Hemosiderin macrophage. [7]
-
Cardiac Myxoma: Gamna Bodies: A peculiar form of fibrosis with deposition of iron pigment, identical to that seen in the spleens of patients with sickle cell anemia, is not uncommon in myxoma.
-
Cardiac Myxoma Common features at the interface with the atrial septum include lymphoid aggregates, smooth muscle bundles, and thick walled vessels which angiographically may look like neovascularization.
-
Cardiac Myxoma The extramedullary hematopoiesis seen here is present in about 7 percent of cardiac myxomas.
-
Cardiac Myxoma Glandular structures are seen in less than 5 percent of cases. In this example, they were limited to the base of the myxoma
Immunohistochemistry
Cardiac myxoma cells exhibit immuno-reactivity mainly for calretinin (75–100%) followed by vimentin (>50%), Notch-1, alpha-1 antichymotrypsin and plakophilin- 2.[8]Calretinin plays an important role in the discrimination of mural thrombi and papillary fibroelastoma.[8]. Another immunohistochemical marker, survivin (an apoptosis inhibitor) has been detected to play an important role in the development and growth of cardiac myxomas.[9]
Genetics
Single cardiac myxomas and familial forms are related with several chromosome and gene alterations which involve cardiac development. Inherited myxomas are usually presented in the Carney complex. The development of this syndrome is a result of PRKAR1A gene inactivation mutation that is associated with chromosome 17q24.2-q24.3. This gene plays an important role in cardiac development and myxomagenesis. The expression of PRKAR1A causes myxomatous changes in the endocardium.[10]
References
- ↑ Bulkley BH, Hutchins GM (1979). "Atrial myxomas: a fifty year review". Am. Heart J. 97 (5): 639–43. PMID 433739.
- ↑ Roscher AA, Kato NS, Quan H, Padmanabhan M (1996). "Intra-atrial myxomas, clinical-pathologic correlation based on two case studies including historical review". J Cardiovasc Surg (Torino). 37 (6 Suppl 1): 131–7. PMID 10064365.
- ↑ Acebo E, Val-Bernal JF, Gómez-Román JJ (2001). "Prichard's structures of the fossa ovalis are not histogenetically related to cardiac myxoma". Histopathology. 39 (5): 529–35. PMID 11737312.
- ↑ 4.0 4.1 Di Vito A, Mignogna C, Donato G (2015). "The mysterious pathways of cardiac myxomas: a review of histogenesis, pathogenesis and pathology". Histopathology. 66 (3): 321–32. doi:10.1111/his.12531. PMID 25297937.
- ↑ Yoon DH, Roberts W (2002). "Sex distribution in cardiac myxomas". Am. J. Cardiol. 90 (5): 563–5. PMID 12208428.
- ↑ Grebenc ML, Rosado de Christenson ML, Burke AP, Green CE, Galvin JR (2000). "Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation". Radiographics. 20 (4): 1073–103, quiz 1110–1, 1112. doi:10.1148/radiographics.20.4.g00jl081073. PMID 10903697.
- ↑ Cardiac Myxoma. Libre Pathology URL http://librepathology.org/wiki/index.php/Cardiac_myxoma Accessed on November 19,2015
- ↑ 8.0 8.1 Acebo E, Val-Bernal JF, Gómez-Roman JJ (2001). "Thrombomodulin, calretinin and c-kit (CD117) expression in cardiac myxoma". Histol. Histopathol. 16 (4): 1031–6. PMID 11642722.
- ↑ Lin YS, Jung SM, Wu HH, Shiu TF, Tzai FC, Chu JJ, Lin PJ, Chu PH (2011). "Survivin expression in cardiac myxoma". Chang Gung Med J. 34 (4): 360–6. PMID 21880190.
- ↑ Sun Y, Chen X, Sun J, Wen X, Liu X, Zhang Y, Hoffman AR, Hu JF, Gao Y (2015). "A Novel Inherited Mutation in PRKAR1A Abrogates PreRNA Splicing in a Carney Complex Family". Can J Cardiol. 31 (11): 1393–401. doi:10.1016/j.cjca.2015.05.018. PMID 26416542.
![1) Black arrow(top):Endothelium 2) Black arrow(bottom): Hemosiderin macrophage. [7]](/images/3/3e/800px-Atrial_myxoma_edge_high_mag.jpg)