Myeloproliferative neoplasm pathophysiology: Difference between revisions
| Line 29: | Line 29: | ||
Genes involved in the pathogenesis of primary myelofibrosis include ''[[Janus kinase 2]]'' ([[JAK2]] kinase) and ''[[Calreticulin]]'' (CALR).<ref name="ganfyd">Ganfyd. Myelofibrosis2015.http://www.ganfyd.org/index.php?title=Primary_myelofibrosis</ref> | Genes involved in the pathogenesis of primary myelofibrosis include ''[[Janus kinase 2]]'' ([[JAK2]] kinase) and ''[[Calreticulin]]'' (CALR).<ref name="ganfyd">Ganfyd. Myelofibrosis2015.http://www.ganfyd.org/index.php?title=Primary_myelofibrosis</ref> | ||
Primary myelofibrosis is hallmarked by clonal myeloproliferation with reactive stromal changes in response to uncontrolled production of growth factors (e.g., [[TGF beta|transforming growth factor β]], [[platelet-derived growth factor|platelet-derived growth factor]], and [[basic fibroblast growth factor|basic fibroblast growth factor]]) from resident [[megakaryocytes]] and [[monocytes]]. Etiopathogenic mutations leading to primary myelofibrosis remain unclear.<ref>{{cite book | last = Jaffe | first = Elaine | title = Pathology and genetics of tumours of haematopoietic and lymphoid tissues | publisher = IARC Press Oxford University Press | year = 2001 | isbn = 978-9283224112 }}</ref> | |||
==References== | ==References== | ||
Revision as of 16:06, 20 October 2015
|
Myeloproliferative Neoplasm Microchapters |
|
Differentiating myeloproliferative neoplasm from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Myeloproliferative neoplasm pathophysiology On the Web |
|
American Roentgen Ray Society Images of Myeloproliferative neoplasm pathophysiology |
|
Directions to Hospitals Treating Myeloproliferative neoplasm |
|
Risk calculators and risk factors for Myeloproliferative neoplasm pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]Associate Editor(s)-in-Chief: Mohamad Alkateb, MBBCh [2]
Overview
Clinical and pathologic features in the myeloproliferative neoplasms are due to dysregulated proliferation and expansion of myeloid progenitors in the bone marrow, resulting in altered populations of granulocytes, erythrocytes, or platelets in the peripheral blood.
Pathophysiology
 |
Genetics
Primary cytogenetic abnormalities have not been identified in the majority of myeloproliferative neoplasms. Aberrant activation of tyrosine kinases and associated signaling pathways is frequently implicated as the disease-initiating event.
Chronic Myelogenous Leukemia
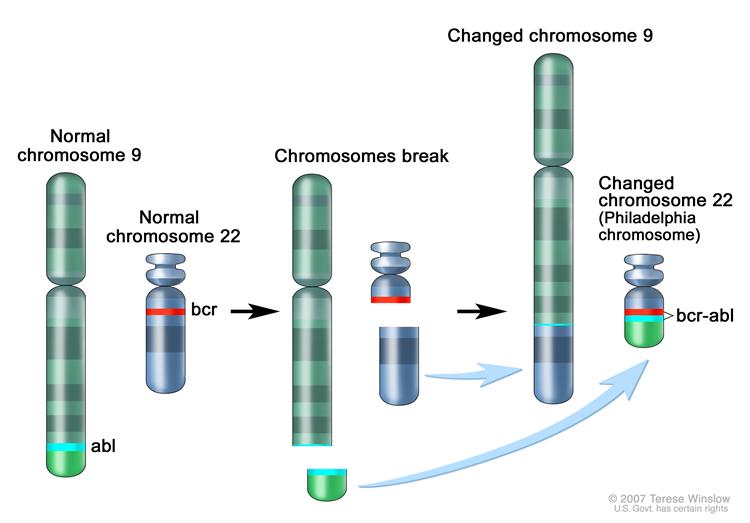
In Philadelphia chromosome translocation, parts of two chromosomes (the 9th and 22nd by conventional karyotypic numbering) switch places. As a result, part of the BCR ("breakpoint cluster region") gene from chromosome 22 is fused with the ABL gene on chromosome 9. This abnormal "fusion" gene generates a protein of p210 or sometimes p185 weight (p is a weight measure of cellular proteins in kDa). Because abl carries a domain that can add phosphate groups to tyrosine residues (a tyrosine kinase), the BCR-ABL fusion gene product is also a tyrosine kinase. The fused BCR-ABL protein interacts with the interleukin 3beta c receptor subunit. The BCR-ABL transcript is continuously active and does not require activation by other cellular messaging proteins. In turn BCR-ABL activates a cascade of proteins which control the cell cycle, speeding up cell division. Moreover the bcr-abl protein inhibits DNA repair, causing genomic instability and making the cell more susceptible to developing further genetic abnormalities. The action of the BCR-ABL protein is the pathophysiologic cause of chronic myelogenous leukemia.[2]
Polycythemia Vera
- Gene involved in the pathogenesis of polycythemia vera is Janus kinase 2 (JAK2 kinase) will lead to activation of the following pathways:[3]
- JAK-STAT
- PI3K (phosphoinositide-3-kinase)
- AKT (protein kinase B)
- The activation of these pathways causes neoplastic proliferation and maturation of erythroid cells.
Essential Thrombocythemia
Calreticulin gene (CALR) gene involved in the pathogenesis of essential thrombocythemia.[4]
Primary Myelofibrosis
Genes involved in the pathogenesis of primary myelofibrosis include Janus kinase 2 (JAK2 kinase) and Calreticulin (CALR).[3]
Primary myelofibrosis is hallmarked by clonal myeloproliferation with reactive stromal changes in response to uncontrolled production of growth factors (e.g., transforming growth factor β, platelet-derived growth factor, and basic fibroblast growth factor) from resident megakaryocytes and monocytes. Etiopathogenic mutations leading to primary myelofibrosis remain unclear.[5]
References
- ↑ "Chronic Myelogenous Leukemia Treatment".
- ↑ Hehlmann R, Hochhaus A, Baccarani M; European LeukemiaNet (2007). "Chronic myeloid leukaemia". Lancet. 370 (9584): 342–50. PMID 17662883.
- ↑ 3.0 3.1 Ganfyd. Polycythaemia vera 2015.http://www.ganfyd.org/index.php?title=Polycythemia_vera
- ↑ Schmoldt A, Benthe HF, Haberland G (1975). "Digitoxin metabolism by rat liver microsomes". Biochem Pharmacol. 24 (17): 1639–41. PMID http://dx.doi.org/10.1182/blood-2013-11-538983 Check
|pmid=value (help). - ↑ Jaffe, Elaine (2001). Pathology and genetics of tumours of haematopoietic and lymphoid tissues. IARC Press Oxford University Press. ISBN 978-9283224112.