Lidocaine/Prilocaine (subgengival): Difference between revisions
(Created page with "{{DrugProjectFormSinglePage |indicationType=treatment |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> |blackBoxWarningBody=<i><span style="color:#FF0000...") |
No edit summary |
||
| Line 3: | Line 3: | ||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | ||
|fdaLIADAdult====Indications=== | |||
Oraqix is an amide local anesthetic indicated for adults who require localized anesthesia in periodontal pockets during scaling and/or root planing. | |||
===Dosage=== | |||
2.1 General Dosing Information | |||
DO NOT INJECT [see WARNINGS AND PRECAUTIONS (5.2)] | |||
Apply Oraqix on the gingival margin around the selected teeth using the blunt-tipped applicator included in the package. Wait 30 seconds, and then fill the periodontal pockets with Oraqix using the blunt-tipped applicator until the gel becomes visible at the gingival margin. Wait another 30 seconds before starting treatment. A longer waiting time does not enhance the anesthesia. Anesthetic effect, as assessed by probing of pocket depths, has a duration of approximately 20 minutes (individual overall range 14 – 31 minutes). If the anesthesia starts to wear off, Oraqix may be re-applied if needed. | |||
Typically, 1 cartridge (1.7g) or less of Oraqix will be sufficient for one quadrant of the dentition. | |||
When administered, Oraqix should be a liquid. If it has formed a gel, it should be placed in a refrigerator (do not freeze) until it becomes a liquid again. When in the liquid state, the air bubble visible in the cartridge will move if the cartridge is tilted. | |||
2.2 Maximum Recommended Dosage | |||
The maximum recommended dose of Oraqix at one treatment session is 5 cartridges, i.e., 8.5g gel. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Lidocaine/Prilocaine (subgengival) in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Lidocaine/Prilocaine (subgengival) in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Lidocaine/Prilocaine (subgengival) in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Lidocaine/Prilocaine (subgengival) in adult patients. | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Lidocaine/Prilocaine (subgengival) in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Lidocaine/Prilocaine (subgengival) in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Lidocaine/Prilocaine (subgengival) in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Lidocaine/Prilocaine (subgengival) in pediatric patients. | ||
|contraindications=Oraqix is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type or to any other component of the product. | |||
|warnings=5.1 Methemoglobinemia | |||
Prilocaine in Oraqix can cause elevated methemoglobin levels particularly in conjunction with methemoglobin-inducing agents. Methemoglobinemia has also been reported in a few cases in association with lidocaine treatment. Patients with glucose-6-phosphate dehydrogenase deficiency or congenital or idiopathic methemoglobinemia are more susceptible to drug-induced methemoglobinemia. Oraqix should not be used in those patients with congenital or idiopathic methemoglobinemia and in infants under the age of twelve months who are receiving treatment with methemoglobin-inducing agents. Signs and symptoms of methemoglobinemia may be delayed some hours after exposure. Initial signs and symptoms of methemoglobinemia are characterized by a slate grey cyanosis seen in, e.g., buccal mucous membranes, lips and nail beds. In severe cases symptoms may include central cyanosis, headache, lethargy, dizziness, fatigue, syncope, dyspnea, CNS depression, seizures, dysrhythmia and shock. Methemoglobinemia should be considered if central cyanosis unresponsive to oxygen therapy occurs, especially if metHb-inducing agents have been used. Calculated oxygen saturation and pulse oximetry are inaccurate in the setting of methemoglobinemia. The diagnosis can be confirmed by an elevated methemoglobin level measured with co-oximetry. Normally, metHb levels are <1%, and cyanosis may not be evident until a level of at least 10% is present. The development of methemoglobinemia is generally dose related. The individual maximum level of metHb in blood ranged from 0.8% to 1.7% following administration of the maximum dose of 8.5g Oraqix. | |||
Management of Methemoglobinemia: Clinically significant symptoms of methemoglobinemia should be treated with a standard clinical regimen such as a slow intravenous infection of methylene blue at a dosage of 1to 2 mg/kg given over a five minute period. | |||
Patients taking drugs associated with drug-induced methemoglobinemia such as sulfonamides, acetaminophen, acetanilide, aniline dyes, benzocaine, chloroquine, dapsone, naphthalene, nitrates and nitrites, nitrofurantoin, nitroglycerin, nitroprusside, pamaquine, para-aminosalicylic acid, phenacetin, phenobarbital, phenytoin, primaquine, and quinine are also at greater risk for developing methemoglobinemia. Treatment with Oraqix should be avoided in patients with any of the above conditions or with a previous history of problems in connection with prilocaine treatment. | |||
5.2 DO NOT INJECT | |||
Oraqix should not be used with standard dental syringes. Only use this product with the Oraqix blunt-tipped applicator, which is available from DENTSPLY Pharmaceutical. | |||
5.3 Allergic/anaphylactic reactions | |||
Allergic and anaphylactic reactions associated with lidocaine or prilocaine in Oraqix can occur. These reactions may be characterized by urticaria, angioedema, bronchospasm, and shock. If these reactions occur they should be managed by conventional means. | |||
5.4 Avoid Contact with Eyes | |||
Oraqix coming in contact with the eye should be avoided because animal studies have demonstrated severe eye irritation. A loss of protective reflexes may allow corneal irritation and potential abrasion. If eye contact occurs, immediately rinse the eye with water or saline and protect it until normal sensation returns. In addition, the patient should be evaluated by an ophthalmologist, as indicated. | |||
5.5 History of Drug Sensitivity | |||
Patients allergic to paraminobenzoic acid derivatives (procaine, tetracaine, benzocaine, etc.) have not shown cross sensitivity to lidocaine and/or prilocaine. However, Oraqix should be used with caution in patients with a history of drug sensitivities, especially if the etiologic agent is uncertain. | |||
5.6 Severe Hepatic Disease | |||
Patients with severe hepatic disease, because of their inability to metabolize local anesthetics normally, are at greater risk of developing toxic plasma concentrations of lidocaine and prilocaine. | |||
|clinicalTrials=Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | |||
Although no major differences in adverse events between Oraqix and placebo-treated subjects were observed, all patients in the placebo-controlled studies received either Oraqix or a placebo gel (consisting of the vehicle in Oraqix without lidocaine or prilocaine). Therefore, it is not possible to determine if adverse events in each treatment group were attributable to the inactive ingredients comprising the Oraqix or vehicle or if adverse event rates were higher than expected background rates. Therefore, a causal relationship between the reported adverse reactions and Oraqix could neither be established nor ruled out. | |||
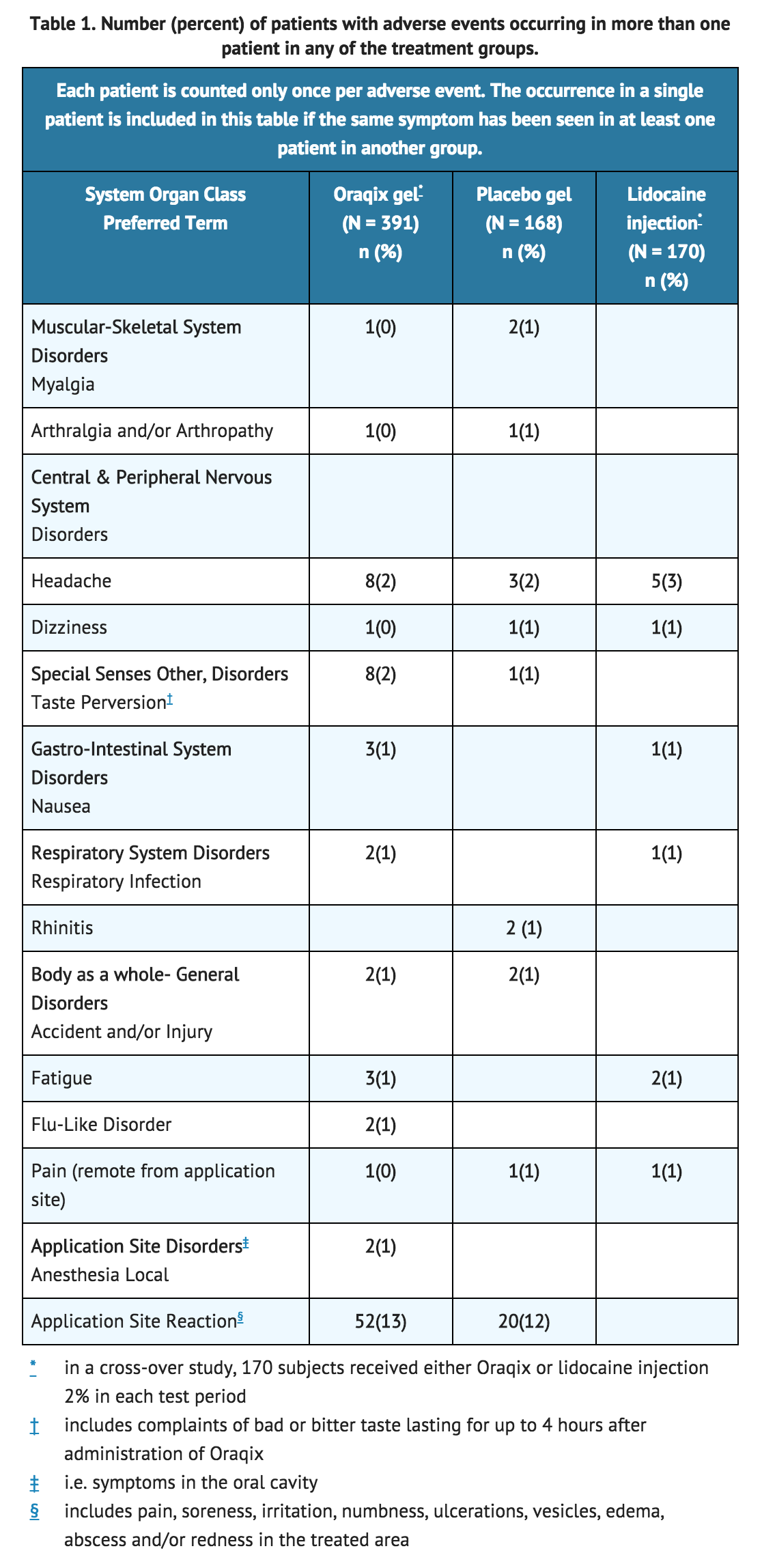
Following SRP treatment with Oraqix in 391 patients, the most frequent adverse events were local reactions in the oral cavity (see following TABLE). These events, which occurred in approximately 15% of patients, included pain, soreness, irritation, numbness, vesicles, ulcerations, edema and/or redness in the treated area. Of the 391 patients treated with Oraqix, five developed ulcerative lesions and two developed vesicles of mild to moderate severity near the site of SRP. In addition, ulcerative lesions in or near the treated area were also reported for three out of 168 patients who received placebo. Other symptoms reported in more than one patient were headache, taste perversion, nausea, fatigue, flu, respiratory infection, musculoskeletal pain and accident/injury. | |||
[[File:ORAQIX1.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|alcohol=Alcohol-Lidocaine/Prilocaine (subgengival) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Lidocaine/Prilocaine (subgengival) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 22:19, 24 May 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Lidocaine/Prilocaine (subgengival) is {{{aOrAn}}} {{{drugClass}}} that is FDA approved for the treatment of {{{indication}}}. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Oraqix is an amide local anesthetic indicated for adults who require localized anesthesia in periodontal pockets during scaling and/or root planing.
Dosage
2.1 General Dosing Information DO NOT INJECT [see WARNINGS AND PRECAUTIONS (5.2)]
Apply Oraqix on the gingival margin around the selected teeth using the blunt-tipped applicator included in the package. Wait 30 seconds, and then fill the periodontal pockets with Oraqix using the blunt-tipped applicator until the gel becomes visible at the gingival margin. Wait another 30 seconds before starting treatment. A longer waiting time does not enhance the anesthesia. Anesthetic effect, as assessed by probing of pocket depths, has a duration of approximately 20 minutes (individual overall range 14 – 31 minutes). If the anesthesia starts to wear off, Oraqix may be re-applied if needed.
Typically, 1 cartridge (1.7g) or less of Oraqix will be sufficient for one quadrant of the dentition.
When administered, Oraqix should be a liquid. If it has formed a gel, it should be placed in a refrigerator (do not freeze) until it becomes a liquid again. When in the liquid state, the air bubble visible in the cartridge will move if the cartridge is tilted.
2.2 Maximum Recommended Dosage The maximum recommended dose of Oraqix at one treatment session is 5 cartridges, i.e., 8.5g gel.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Lidocaine/Prilocaine (subgengival) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Lidocaine/Prilocaine (subgengival) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Lidocaine/Prilocaine (subgengival) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Lidocaine/Prilocaine (subgengival) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Lidocaine/Prilocaine (subgengival) in pediatric patients.
Contraindications
Oraqix is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type or to any other component of the product.
Warnings
5.1 Methemoglobinemia Prilocaine in Oraqix can cause elevated methemoglobin levels particularly in conjunction with methemoglobin-inducing agents. Methemoglobinemia has also been reported in a few cases in association with lidocaine treatment. Patients with glucose-6-phosphate dehydrogenase deficiency or congenital or idiopathic methemoglobinemia are more susceptible to drug-induced methemoglobinemia. Oraqix should not be used in those patients with congenital or idiopathic methemoglobinemia and in infants under the age of twelve months who are receiving treatment with methemoglobin-inducing agents. Signs and symptoms of methemoglobinemia may be delayed some hours after exposure. Initial signs and symptoms of methemoglobinemia are characterized by a slate grey cyanosis seen in, e.g., buccal mucous membranes, lips and nail beds. In severe cases symptoms may include central cyanosis, headache, lethargy, dizziness, fatigue, syncope, dyspnea, CNS depression, seizures, dysrhythmia and shock. Methemoglobinemia should be considered if central cyanosis unresponsive to oxygen therapy occurs, especially if metHb-inducing agents have been used. Calculated oxygen saturation and pulse oximetry are inaccurate in the setting of methemoglobinemia. The diagnosis can be confirmed by an elevated methemoglobin level measured with co-oximetry. Normally, metHb levels are <1%, and cyanosis may not be evident until a level of at least 10% is present. The development of methemoglobinemia is generally dose related. The individual maximum level of metHb in blood ranged from 0.8% to 1.7% following administration of the maximum dose of 8.5g Oraqix.
Management of Methemoglobinemia: Clinically significant symptoms of methemoglobinemia should be treated with a standard clinical regimen such as a slow intravenous infection of methylene blue at a dosage of 1to 2 mg/kg given over a five minute period.
Patients taking drugs associated with drug-induced methemoglobinemia such as sulfonamides, acetaminophen, acetanilide, aniline dyes, benzocaine, chloroquine, dapsone, naphthalene, nitrates and nitrites, nitrofurantoin, nitroglycerin, nitroprusside, pamaquine, para-aminosalicylic acid, phenacetin, phenobarbital, phenytoin, primaquine, and quinine are also at greater risk for developing methemoglobinemia. Treatment with Oraqix should be avoided in patients with any of the above conditions or with a previous history of problems in connection with prilocaine treatment.
5.2 DO NOT INJECT Oraqix should not be used with standard dental syringes. Only use this product with the Oraqix blunt-tipped applicator, which is available from DENTSPLY Pharmaceutical.
5.3 Allergic/anaphylactic reactions Allergic and anaphylactic reactions associated with lidocaine or prilocaine in Oraqix can occur. These reactions may be characterized by urticaria, angioedema, bronchospasm, and shock. If these reactions occur they should be managed by conventional means.
5.4 Avoid Contact with Eyes Oraqix coming in contact with the eye should be avoided because animal studies have demonstrated severe eye irritation. A loss of protective reflexes may allow corneal irritation and potential abrasion. If eye contact occurs, immediately rinse the eye with water or saline and protect it until normal sensation returns. In addition, the patient should be evaluated by an ophthalmologist, as indicated.
5.5 History of Drug Sensitivity Patients allergic to paraminobenzoic acid derivatives (procaine, tetracaine, benzocaine, etc.) have not shown cross sensitivity to lidocaine and/or prilocaine. However, Oraqix should be used with caution in patients with a history of drug sensitivities, especially if the etiologic agent is uncertain.
5.6 Severe Hepatic Disease Patients with severe hepatic disease, because of their inability to metabolize local anesthetics normally, are at greater risk of developing toxic plasma concentrations of lidocaine and prilocaine.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Although no major differences in adverse events between Oraqix and placebo-treated subjects were observed, all patients in the placebo-controlled studies received either Oraqix or a placebo gel (consisting of the vehicle in Oraqix without lidocaine or prilocaine). Therefore, it is not possible to determine if adverse events in each treatment group were attributable to the inactive ingredients comprising the Oraqix or vehicle or if adverse event rates were higher than expected background rates. Therefore, a causal relationship between the reported adverse reactions and Oraqix could neither be established nor ruled out.
Following SRP treatment with Oraqix in 391 patients, the most frequent adverse events were local reactions in the oral cavity (see following TABLE). These events, which occurred in approximately 15% of patients, included pain, soreness, irritation, numbness, vesicles, ulcerations, edema and/or redness in the treated area. Of the 391 patients treated with Oraqix, five developed ulcerative lesions and two developed vesicles of mild to moderate severity near the site of SRP. In addition, ulcerative lesions in or near the treated area were also reported for three out of 168 patients who received placebo. Other symptoms reported in more than one patient were headache, taste perversion, nausea, fatigue, flu, respiratory infection, musculoskeletal pain and accident/injury.
Postmarketing Experience
There is limited information regarding Lidocaine/Prilocaine (subgengival) Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Lidocaine/Prilocaine (subgengival) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Lidocaine/Prilocaine (subgengival) in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Lidocaine/Prilocaine (subgengival) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Lidocaine/Prilocaine (subgengival) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Lidocaine/Prilocaine (subgengival) in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Lidocaine/Prilocaine (subgengival) in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Lidocaine/Prilocaine (subgengival) in geriatric settings.
Gender
There is no FDA guidance on the use of Lidocaine/Prilocaine (subgengival) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Lidocaine/Prilocaine (subgengival) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Lidocaine/Prilocaine (subgengival) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Lidocaine/Prilocaine (subgengival) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Lidocaine/Prilocaine (subgengival) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Lidocaine/Prilocaine (subgengival) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Lidocaine/Prilocaine (subgengival) Administration in the drug label.
Monitoring
There is limited information regarding Lidocaine/Prilocaine (subgengival) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Lidocaine/Prilocaine (subgengival) and IV administrations.
Overdosage
There is limited information regarding Lidocaine/Prilocaine (subgengival) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Lidocaine/Prilocaine (subgengival) Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Lidocaine/Prilocaine (subgengival) Mechanism of Action in the drug label.
Structure
There is limited information regarding Lidocaine/Prilocaine (subgengival) Structure in the drug label.
Pharmacodynamics
There is limited information regarding Lidocaine/Prilocaine (subgengival) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Lidocaine/Prilocaine (subgengival) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Lidocaine/Prilocaine (subgengival) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Lidocaine/Prilocaine (subgengival) Clinical Studies in the drug label.
How Supplied
There is limited information regarding Lidocaine/Prilocaine (subgengival) How Supplied in the drug label.
Storage
There is limited information regarding Lidocaine/Prilocaine (subgengival) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Lidocaine/Prilocaine (subgengival) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Lidocaine/Prilocaine (subgengival) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Lidocaine/Prilocaine (subgengival) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Lidocaine/Prilocaine (subgengival) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Lidocaine/Prilocaine (subgengival) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Lidocaine/Prilocaine (subgengival) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.