Leucovorin (injection)
 | |
| Clinical data | |
|---|---|
| Pregnancy category | |
| Routes of administration | Intravenous, oral |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | Dose dependent:
|
| Protein binding | ~15% |
| Elimination half-life | 6.2 hours |
| Excretion | Urinary |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
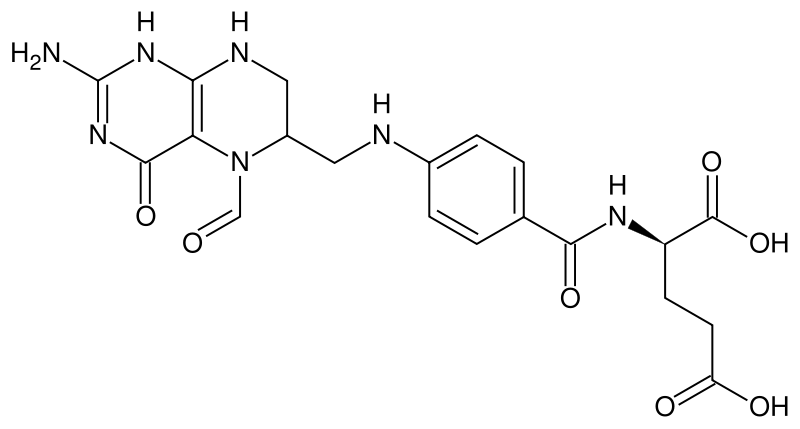
| Formula | C20H23N7O7 |
| Molar mass | 473.44 g/mol |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Folinic acid (INN) or leucovorin (USAN), generally administered as calcium folinate (or leucovorin calcium), is an adjuvant used in cancer chemotherapy involving the drug methotrexate. It is also used in synergistic combination with the chemotherapy agent 5-fluorouracil.
Folinic acid is not folic acid.
Mechanism of action
Folinic acid is a 5-formyl derivative of tetrahydrofolic acid. It is readily converted to other reduced folic acid derivatives (e.g. tetrahydrofolate), and thus has vitamin activity which is equivalent to folic acid. However, since it does not require the action of dihydrofolate reductase for its conversion, its function as a vitamin is unaffected by inhibition of this enzyme by drugs such as methotrexate.
Folinic acid, therefore, allows for some purine/pyrimidine synthesis to occur in the presence of dihydrofolate reductase inhibition, so that some normal DNA replication and RNA transcription processes can proceed.
Therapeutic use
Folinic acid is administered at the appropriate time following methotrexate as part of a total chemotherapeutic plan, where it may "rescue" bone marrow and gastrointestinal mucosa cells from methotrexate. There is no apparent effect on preexisting methotrexate-induced nephrotoxicity.[1]
While not specifically an antidote for methotrexate, folinic acid may also be useful in the treatment of acute methotrexate overdose. Different dosing protocols are used, but folinic acid should be re-dosed until the methotrexate level is less than 5 x 10-8 M (this can also be re-stated as < 0.05 μM) [2]
Folinic acid is also used in combination with the chemotherapy agent 5-fluorouracil in treating colon cancer. In this case, folinic acid is not used for "rescue" purposes; rather, it enhances the effect of 5-fluorouracil on inhibiting thymidylate synthase.
Folinic acid is also sometimes used to prevent toxic effects of high doses of antimicrobial dihydrofolate reductase inhibitors such as trimethoprim and pyrimethamine.
Note on administration
Folinic acid should not be administered intrathecally. This may produce severe adverse effects or even death.[3]
References
- ↑ Therapeutic Information Resources Australia (2004). Calcium Folinate (Systemic) in AUSDI: Australian Drug Information for the Health Care Professional. Castle Hill: Therapeutic Information Resources Australia.
- ↑ www.cancercare.on.ca/pdfdrugs/leucovo.pdf
- ↑ Jardine, LF; et al. (1996). "Intrathecal Leucovorin After Intrathecal Methotrexate Overdose". J Pediatr Hematol Oncol. 18 (3): 302&ndash, 304. PMID 8689347.
Template:Detoxifying agents for antineoplastic treatment Template:SIB
- Pages with script errors
- CS1 maint: Explicit use of et al.
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Chemotherapeutic adjuvants
- Folates