Lanadelumab
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Zach Leibowitz [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Lanadelumab is a plasma kallikrein inhibitor (monoclonal antibody) that is FDA approved for the prophylaxis to prevent attacks of hereditary angioedema (HAE) in patients 12 years and older. Common adverse reactions include injection site reactions, upper respiratory infections, headache, rash, myalgia, dizziness, and diarrhea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Lanadelumab is indicated for prophylaxis to prevent attacks of hereditary angioedema (HAE) in patients 12 years and older.
Dosage
- The recommended starting dose is 300 mg every 2 weeks. A dosing interval of 300 mg every 4 weeks is also effective and may be considered if the patient is well-controlled (e.g., attack free) for more than 6 months.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding lanadelumab Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding lanadelumab Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indication
- Lanadelumab is indicated for prophylaxis to prevent attacks of hereditary angioedema (HAE) in patients 12 years and older.
- The safety and efficacy of lanadelumab in pediatric patients < 12 years of age have not been established.
Dosage
- The recommended starting dose for pediatric patients 12 years or older is 300 mg every 2 weeks. A dosing interval of 300 mg every 4 weeks is also effective and may be considered if the patient is well-controlled (e.g., attack free) for more than 6 months.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding lanadelumab Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding lanadelumab Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
None.
Warnings
Hypersensitivity Reactions
- Hypersensitivity reactions have been observed. In case of a severe hypersensitivity reaction, discontinue lanadelumab administration and institute appropriate treatment.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety of lanadelumab is primarily based on a 26-week, randomized, double-blind, parallel-group and placebo-controlled study (Trial 1) in 125 patients with Type I or II HAE. Eligible patients were also able to participate in an open-label extension study (Trial 2) up to 130 weeks. In Trial 1, a total of 84 patients with HAE aged 12 years and older received at least one dose of lanadelumab. Overall, 70% of patients were female and 90% of patients were Caucasian with a mean age of 41 years. The proportion of patients who discontinued study drug prematurely due to adverse events was 1.2% for lanadelumab-treated patients and 4.9% for placebo-treated patients. No deaths occurred in the trial.
- The safety profile of lanadelumab was generally similar across all subgroups of patients, including analysis by age, sex, and geographic region.
- Table 1 shows adverse reactions occurring in ≥10% of patients in any lanadelumab treatment group that also occurred at a higher rate than in the placebo treatment group in Trial 1.

- Injection site reactions primarily consisted mainly of pain, erythema, and bruising at the injection site. There was no meaningful difference in injection site reactions with self-administration.
Less Common Adverse Reactions
- Other adverse reactions that occurred at a higher incidence in lanadelumab-treated patients compared to placebo include hypersensitivity (1% vs 0%), increased aspartate transaminase (2% vs 0%), and increased alanine transaminase (2% vs 0%).
- Safety data from the ongoing open-label extension study, consisting of 109 rollover patients from Trial 1 and 103 non-rollover HAE patients, is consistent with controlled safety data from Trial 1.
Laboratory Abnormalities
Transaminase elevations
- During the placebo-controlled treatment period in Trial 1, the number of lanadelumab-treated patients with maximum transaminase (ALT or AST) levels >8, >5, or >3 times the upper limit of normal (ULN) was 1 (1.2%), 0 (0%), or 3 (3.6%) respectively, compared to 0 in the placebo-treated patients. These transaminase elevations were asymptomatic and transient. No patients had elevated total bilirubin >2× ULN. One lanadelumab-treated patient permanently discontinued treatment due to elevated transaminases (4.1× ULN AST). None of the patients were reported to have serious adverse reactions of elevated transaminases.
Immunogenicity
- As with all therapeutic proteins, there is a potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to lanadelumab-flyo in the study described below with the incidence of antibodies in other studies or to other products may be misleading.
- In Trial 1, 10 (12%) lanadelumab-flyo-treated and 2 (5%) placebo-treated patients had at least 1 anti-drug antibody (ADA)-positive sample during the treatment period; antibody titers were low (range: 20 to 1280). The ADA response observed was transient in 2/10 lanadelumab-flyo and 1/2 placebo-treated patients. Pre-existing low titer antibodies were observed in 3 lanadelumab-flyo-treated patients and 1 placebo-treated patient with ADAs. Two patients receiving 150 mg q4wks had low titer antibodies classified as neutralizing.
- The development of ADA including neutralizing antibodies against lanadelumab-flyo did not appear to adversely affect pharmacokinetics (PK), pharmacodynamics (PD), safety or clinical response.
Postmarketing Experience
There is limited information regarding Lanadelumab Postmarketing Experience in the drug label.
Drug Interactions
- No dedicated drug interaction studies have been conducted.
Drug-Laboratory Test Interactions
Coagulation tests
- Lanadelumab can increase activated partial thromboplastin time (aPTT) due to an interaction of lanadelumab with the aPTT assay. The reagents used in the aPTT laboratory test initiate intrinsic coagulation through the activation of plasma kallikrein in the contact system. Inhibition of plasma kallikrein by lanadelumab can increase aPTT in this assay. In Trial 1, prolongation of aPTT (>1× ULN) was observed at one or more time points in 3, 9, and 11 patients treated with lanadelumab 150 mg q4 wks, 300 mg q4 wks, and 300 mg q2 wks, respectively, compared to 5 placebo-treated patients. Only one patient in the 300 mg q2 wks treatment group experienced transient aPTT prolongation ≥1.5× ULN which was confounded by ongoing heparin therapy. None of the increases in aPTT in patients treated with lanadelumab were associated with abnormal bleeding adverse events. There were no differences in INR values between treatment groups.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Risk Summary
- There are no available data on lanadelumab use in pregnant women to inform any drug associated risks. Monoclonal antibodies such as lanadelumab-flyo are transported across the placenta during the third trimester of pregnancy; therefore, potential effects on a fetus are likely to be greater during the third trimester of pregnancy. An enhanced pre-and postnatal development (ePPND) study conducted in pregnant monkeys at doses resulting in exposures of up to 33 times the exposure achieved (on an AUC basis) at the maximum recommended human dose (MRHD) revealed no evidence of harm to the developing fetus.
- The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Animal Data
- In the ePPND study, pregnant cynomolgus monkeys were administered lanadelumab-flyo once weekly at subcutaneous doses resulting in up to 33 times the exposure at the MRHD (on an AUC basis with maternal subcutaneous doses up to 50 mg/kg/week) from gestation day 20, at the beginning of organogenesis, through to parturition. There were no lanadelumab-flyo-related effects on maintenance of pregnancy or parturition. Maternal lanadelumab-flyo treatment had no effects on embryo-fetal development, survival, growth, or postnatal development of offspring through 3 months of age. Lanadelumab-flyo crossed the placenta in monkeys. Offspring were exposed to lanadelumab-flyo at approximately 50% of the maternal plasma concentration out to postnatal day 21 (PND 21). Lanadelumab-flyo concentrations were approximately equivalent in maternal and offspring plasma at PND 90.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Lanadelumab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Lanadelumab during labor and delivery.
Nursing Mothers
Risk Summary
- There are no data on the presence of lanadelumab-flyo in human milk, its effects on the breastfed infant, or its effects on milk production. Lanadelumab-flyo was detected in the milk of lactating cynomolgus monkeys at approximately 0.2% of the maternal plasma concentration. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for lanadelumab and any potential adverse effects on the breastfed infant from lanadelumab or from the underlying maternal condition.
Animal Data
- Available pharmacokinetic data in cynomolgus monkeys have shown excretion of lanadelumab-flyo in milk at approximately 0.2% of the maternal plasma level.
Pediatric Use
- The safety and efficacy of lanadelumab were evaluated in a subgroup of patients (N=10) aged 12 to <18 years in Trial 1. Results of the subgroup analysis by age were consistent with overall study results. An additional 13 adolescent patients aged 12 to <18 years were enrolled in the open-label extension study.
- The safety and efficacy of lanadelumab in pediatric patients < 12 years of age have not been established.
Geriatic Use
- The safety and efficacy of lanadelumab were evaluated in a subgroup of patients (N=5) aged ≥65 years in Trial 1. Results of the subgroup analysis by age were consistent with overall study results.
Gender
There is no FDA guidance on the use of Lanadelumab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Lanadelumab with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Lanadelumab in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Lanadelumab in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Lanadelumab in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Lanadelumab in patients who are immunocompromised.
Administration and Monitoring
Administration
Dosage
- The recommended starting dose is 300 mg every 2 weeks. A dosing interval of 300 mg every 4 weeks is also effective and may be considered if the patient is well-controlled (e.g., attack free) for more than 6 months.
Administration
- Lanadelumab is administered subcutaneously only.
- Lanadelumab is provided as a ready-to-use solution in a single-dose vial that does not require additional reconstitution or dilution for administration. Lanadelumab is supplied as a clear to slightly opalescent, colorless to slightly yellow solution. Do not use the vial if it appears discolored or contains visible particles. Avoid vigorous agitation of the vial.
- Lanadelumab is intended for self-administration or administration by a caregiver. The patient or caregiver should be trained by a healthcare professional.
- Take the lanadelumab vial out of the refrigerator 15 minutes before injecting to allow it to equilibrate to room temperature.
- Using aseptic technique, withdraw the prescribed dose of lanadelumab from the vial using an 18-gauge needle. Change the needle on the syringe to a 27-gauge, ½-inch needle or other needle suitable for subcutaneous injection. Inject lanadelumab subcutaneously into the abdomen, thigh, or upper arm. Patients should inject the complete dose as prescribed by their physician. In clinical studies, the majority of patients self-administered lanadelumab over 10 to 60 seconds.
- Lanadelumab should be administered within 2 hours of preparing the dosing syringe. After the dosing syringe is prepared, it can be refrigerated at 36ºF to 46ºF (2°C to 8°C) and must be used within 8 hours.
- Discard any unused portions of drug remaining in the vial and syringe.
- For detailed instructions on the preparation and administration of lanadelumab see INSTRUCTIONS FOR USE.
Monitoring
There is limited information regarding Lanadelumab Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Lanadelumab and IV administrations.
Overdosage
- There is no clinical experience with overdosage of lanadelumab.
Pharmacology
Mechanism of Action
- Lanadelumab-flyo is a fully human monoclonal antibody (IgG1/κ-light chain) that binds plasma kallikrein and inhibits its proteolytic activity. Plasma kallikrein is a protease that cleaves high-molecular-weight-kininogen (HMWK) to generate cleaved HMWK (cHMWK) and bradykinin, a potent vasodilator that increases vascular permeability resulting in swelling and pain associated with HAE. In patients with HAE due to C1-inhibitor (C1-INH) deficiency or dysfunction, normal regulation of plasma kallikrein activity is not present, which leads to uncontrolled increases in plasma kallikrein activity and results in angioedema attacks. Lanadelumab-flyo decreases plasma kallikrein activity to control excess bradykinin generation in patients with HAE.
Structure
There is limited information regarding Lanadelumab Structure in the drug label.
Pharmacodynamics
- Concentration-dependent inhibition of plasma kallikrein, measured as reduction of cHMWK levels, was demonstrated after subcutaneous administration of lanadelumab 150 mg q4wks, 300 mg q4wks or 300 mg q2wks in patients with HAE.
- Lanadelumab did not prolong the QT/QTc interval.
Pharmacokinetics
- Following subcutaneous administration, the pharmacokinetics of lanadelumab-flyo was approximately dose-proportional in the therapeutic dose range in patients with HAE (Table 2). The pharmacokinetic properties and exposure (steady state) of lanadelumab-flyo in HAE patients, following subcutaneous administration of 150 mg q4wks, 300 mg q4wks and 300 mg q2wks, are provided in Table 2. Following subcutaneous administration of lanadelumab, peak plasma concentrations are reached within 5 days, and terminal elimination half-life is ~2 weeks. The anticipated time to reach steady state concentration was approximately 70 days. At steady-state, the mean accumulation ratio is approximately 1.44, 1.42, and 2.43 for dosing regimen of 150 mg q4wks, 300 mg q4wks and 300 mg q2wks, respectively.

Specific Populations
- Population pharmacokinetic analyses showed that age, gender and race did not meaningfully influence the pharmacokinetics of lanadelumab-flyo after correcting for body weight. Body weight was identified as an important covariate describing the variability of clearance and volume of distribution, resulting in higher exposure (AUC and Cmax) in lighter patients. However, this difference is not considered to be clinically relevant and no dose adjustments are recommended for any of these demographics.
Pediatric Population
- Based on population pharmacokinetics (PK) analyses, the mean lanadelumab-flyo (±SD) AUCss was 629 (204) µg*day/mL following SC administration of lanadelumab 300 mg every 2 weeks in pediatric patients 12 to less than 18 years of age. This is approximately 37% higher than the mean AUCss in adult patients (460 μg*day/mL) under the same dosing regimen, due to lower body weight in pediatric patients.
Renal Impairment
- No dedicated studies have been conducted to evaluate the PK of lanadelumab-flyo in renal impairment patients. Based on population pharmacokinetic analysis, renal impairment (estimated GFR: 60 to 89 mL/min/1.73m2, [mild, N=98] and 30 to 59 mL/min/1.73m2, [moderate, N=9]) had no effect on the clearance or volume of distribution of lanadelumab-flyo.
Concomitant medications
- The use of analgesic, antibacterial, antihistamine, anti-inflammatory and antirheumatic medications had no effect on clearance and volume of distribution of lanadelumab-flyo.
- For breakthrough HAE attacks, use of rescue medications such as plasma-derived and recombinant C1-INH, icatibant or ecallantide had no effects on clearance and volume of distribution of lanadelumab-flyo.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Animal studies have not been conducted to evaluate the carcinogenic potential of lanadelumab-flyo. Published literature supports bradykinin, which is elevated in HAE, as a pro-tumorigenic molecule. However, the malignancy risk in humans from an antibody that inhibits plasma kallikrein activity, such as lanadelumab-flyo, which lowers bradykinin levels, is currently unknown.
- Male and female fertility were unaffected based upon no observed adverse histopathological findings in the reproductive organs from sexually mature cynomolgus monkeys that received lanadelumab-flyo for 13 weeks at subcutaneous doses up to 50 mg/kg/week (resulting in approximately 22 times the exposure at the MRHD on an AUC basis).
Clinical Studies
Trial 1 (NCT02586805)
- The efficacy of lanadelumab for the prevention of angioedema attacks in patients 12 years of age and older with Type I or II HAE was demonstrated in a multicenter, randomized, double-blind, placebo-controlled parallel-group study (Trial 1).
- The study included 125 adult and adolescent patients with Type I or II HAE who experienced at least one investigator-confirmed attack per 4 weeks during the run-in period. Patients were randomized into 1 of 4 parallel treatment arms, stratified by baseline attack rate, in a 3:2:2:2 ratio (placebo, lanadelumab-flyo 150 mg q4wks, lanadelumab-flyo 300 mg q4wks, or lanadelumab-flyo 300 mg q2wks by subcutaneous injection) for the 26-week treatment period. Patients ≥18 years of age were required to discontinue other prophylactic HAE medications prior to entering the study; however, all patients were allowed to use rescue medications for treatment of breakthrough HAE attacks.
- Overall, 90% of patients had Type I HAE. A history of laryngeal angioedema attacks was reported in 65% of patients and 56% were on prior long-term prophylaxis. During the study run-in period, attack rates of ≥3 attacks/month were observed in 52% of patients overall.
- All lanadelumab treatment arms produced clinically meaningful and statistically significant reductions in the mean HAE attack rate compared to placebo across all primary and secondary endpoints in the Intent-to-Treat population (ITT) as shown in Table 3.
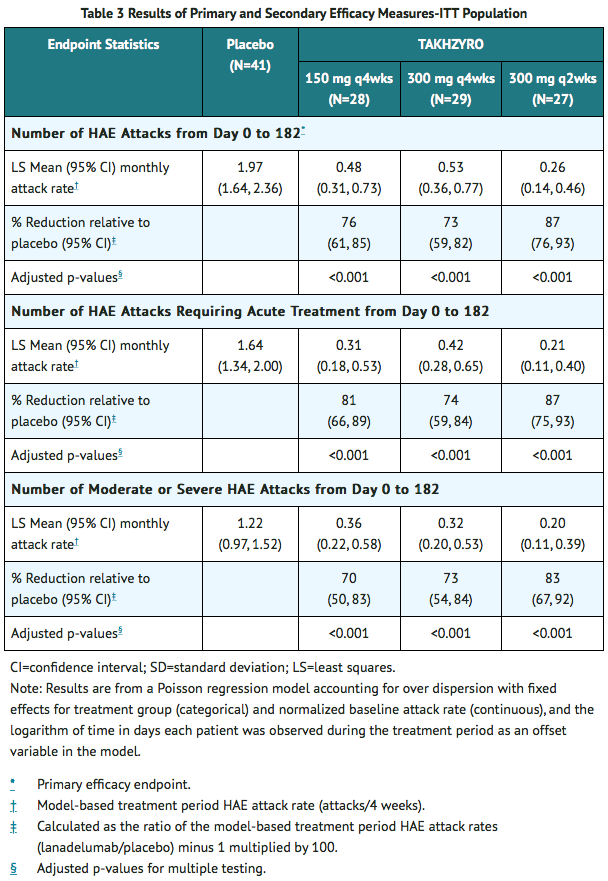
- The mean reduction in HAE attack rate was consistently higher across the lanadelumab treatment arms compared to placebo regardless of the baseline history of prior long-term prophylaxis, laryngeal attacks, or attack rate during the run-in period.
- Additional pre-defined exploratory endpoints included the percentage of patients who were attack free for the entire 26-week treatment period and the percentage of patients achieving threshold (≥50%, ≥70%, ≥90%) reductions in HAE attack rates compared to run-in during the 26-week treatment period. A ≥50% reduction in HAE attack rate was observed in 100% of patients on 300 mg q2wks or q4wks and 89% on 150 mg q4wks compared to 32% of placebo patients. A ≥70% reduction in HAE attack rates was observed in 89%, 76%, and 79% of patients on 300 mg q2wks, 300 mg q4wks, and 150 mg q4wks, respectively, compared to 10% of placebo patients. A ≥90% reduction in HAE attack rates was observed 67%, 55%, and 64% of patients on 300 mg q2wks, 300 mg q4wks, and 150 mg q4wks, respectively, compared to 5% of placebo patients.
- The percentage of attack-free patients for the entire 26-week treatment period was 44%, 31%, and 39% in the lanadelumab 300 mg q2wks, 300 mg q4wks, and 150 mg q4wks groups respectively, compared to 2% of placebo patients.
Trial 2 (NCT02741596)
- Patients who completed Trial 1 were eligible to rollover into an open-label extension study. Rollover patients, regardless of randomization group in Trial 1, received a single dose of lanadelumab 300 mg at study entry and were followed until the first HAE attack occurred. All efficacy endpoints were exploratory in this uncontrolled, unblinded study. At week 4 post-dose, approximately 80% of patients who had been in the 300 mg q2wks treatment group (N=25) in Trial 1 remained attack-free. After the first HAE attack, all patients received open-label treatment with lanadelumab 300 mg q2wks.
How Supplied
- Lanadelumab (lanadelumab-flyo) injection is a ready-to-use, clear to slightly opalescent, colorless to slightly yellow solution supplied in a carton containing one single-dose glass vial with chlorobutyl rubber stopper, aluminum crimp seal and polypropylene flip-off cap.
- 300 mg/2 mL (150 mg/mL) vial.
Storage
- Store vials refrigerated at 36°F to 46°F (2°C to 8°C).
- Do not freeze. Do not shake.
- Keep the vial in the original carton in order to protect the vial from light.
Images
Drug Images
{{#ask: Page Name::Lanadelumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Lanadelumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Hypersensitivity
- Advise patients to seek immediate medical attention if they experience any symptoms of serious hypersensitivity reactions.
Self-administration:
- Ensure that the patient/caregiver receives clear instructions and training on subcutaneous administration and has demonstrated the ability to perform a subcutaneous injection.
- Instruct patients or caregivers in the technique of proper syringe and needle disposal, and advise them not to reuse these items. Instruct patients to dispose needles and syringes in a puncture-resistant container.
Patient Package Insert

Instructions for Use
- Be sure that you read, understand, and follow the Instructions for Use before injecting lanadelumab. A healthcare provider should show you how to prepare and inject lanadelumab properly before you use it for the first time. Contact your healthcare provider if you have any questions.
Important information:
- Lanadelumab is a ready-to-use solution for injection under the skin (subcutaneous). It is supplied in a single-dose, glass vial.
- Your healthcare provider will prescribe the dose that you should take.
- Only use the syringes, transfer needles, and injection needles that your healthcare provider prescribes.
- Only use the syringes, transfer needles and injection needles 1 time. Throw away (dispose of) any used syringes and needles.
Storing lanadelumab:
- Store lanadelumab in the refrigerator at 36°F to 46°F (2°C to 8°C). Do not freeze.
- Store lanadelumab in the original carton to protect the vial from light.
- Do not shake lanadelumab.
- Keep lanadelumab and all medicines out of the reach of children.
Supplies needed for your lanadelumab Injection

STEP 1: Prepare for your injection
- Gather all supplies and place them on a well-lighted flat work surface.
- Take the vial out of the refrigerator 15 minutes before use and allow it to reach room temperature before preparing an injection.
- Check the expiration date on the box and vial label of lanadelumab. Do not use if the expiration date has passed.
- Check the supplies for damage. Do not use if they appear damaged.
- Clean your work area and wash your hands before preparing your dose. Do not touch any surface or body part, especially your face, after washing your hands before injection.
- Remove the vial from the packaging. Do not use the vial if the plastic cap covering is missing.
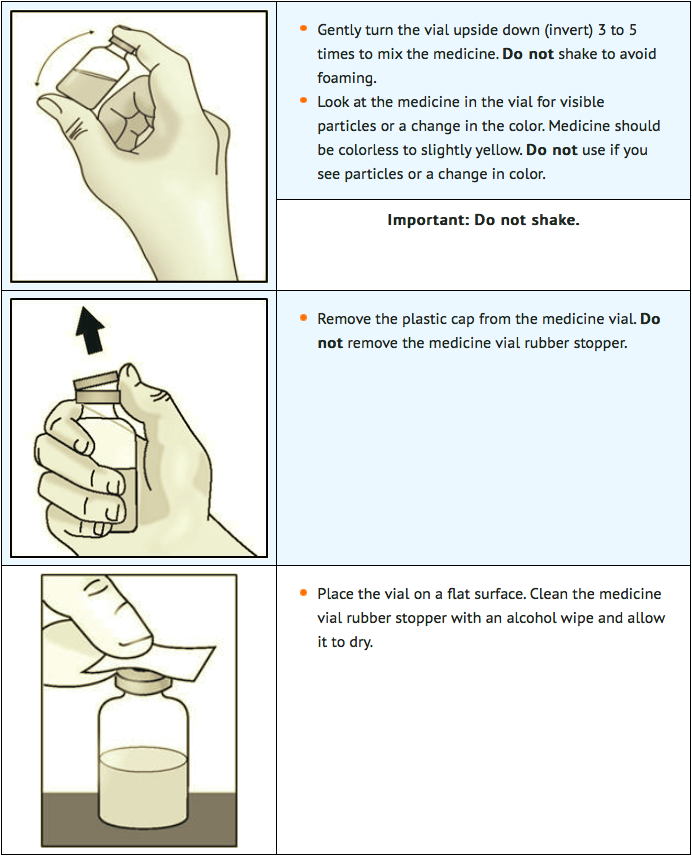
STEP 2: Attach transfer needle to syringe
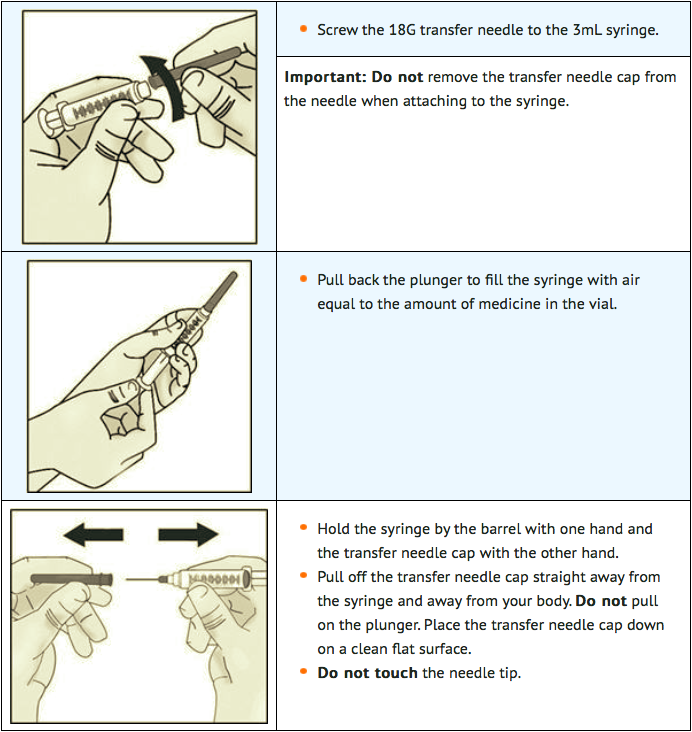
STEP 3: Transfer lanadelumab into syringe and switch to the injection needle
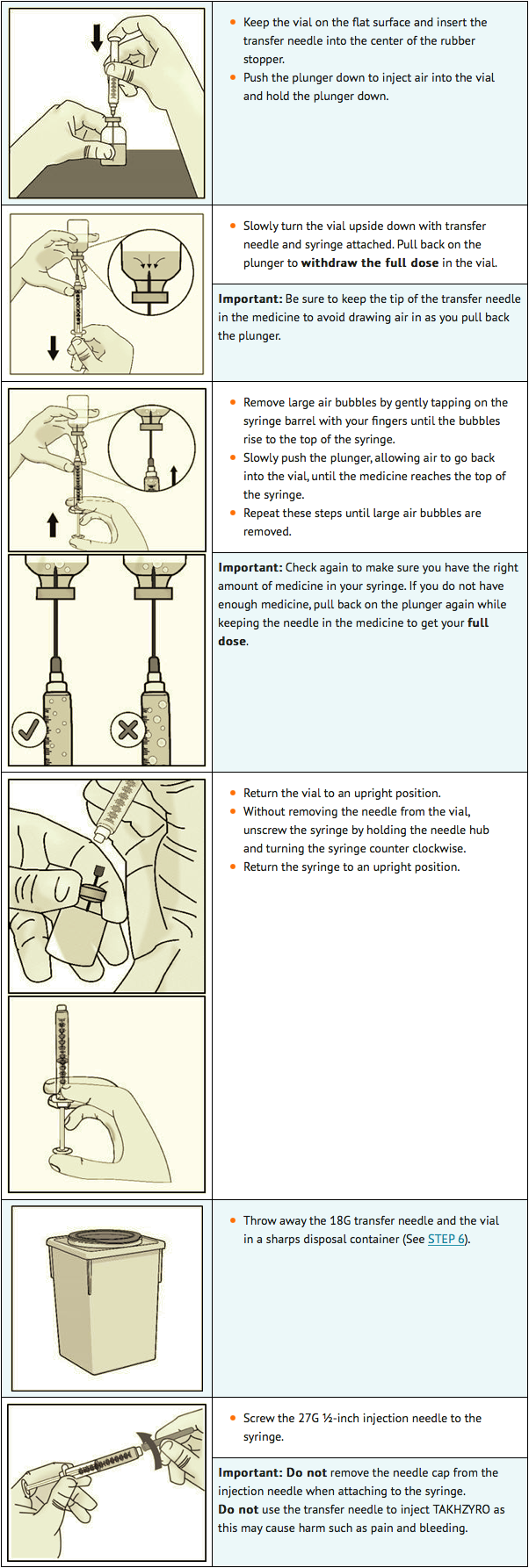
STEP 4: Select and prepare injection site
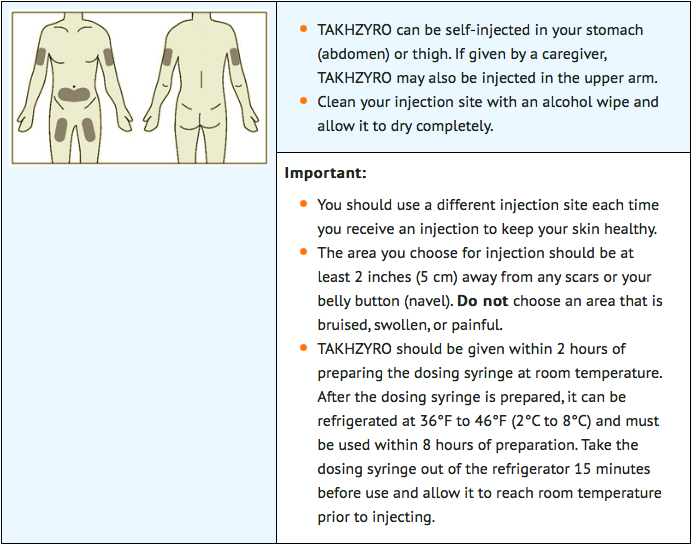
STEP 5: Inject lanadelumab
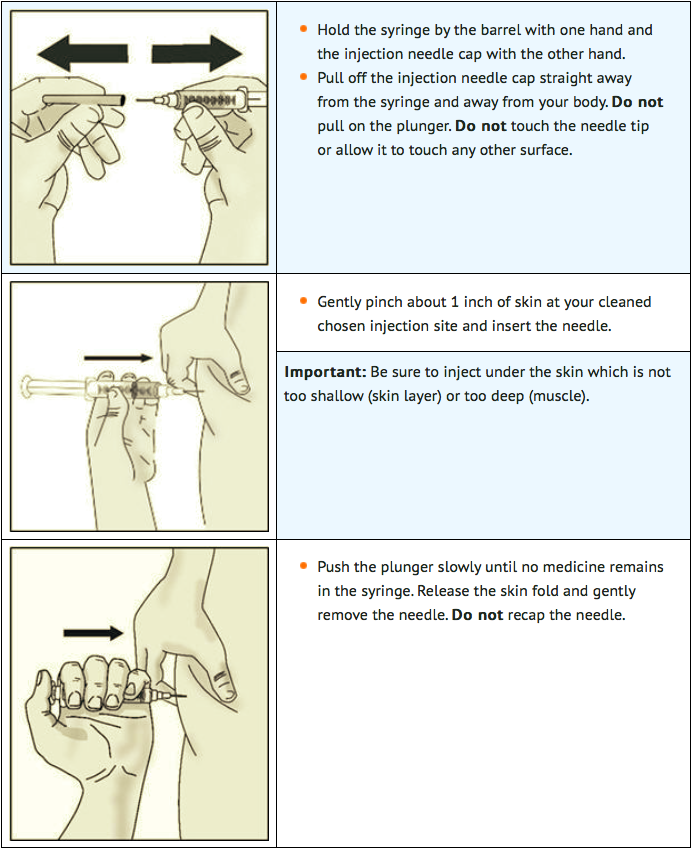
STEP 6: THROW AWAY (DISPOSE OF) NEEDLE AND SYRINGE

- For more information, visit www.takhzyro.com
- This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Precautions with Alcohol
Alcohol-Lanadelumab interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Lanadelumab Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.