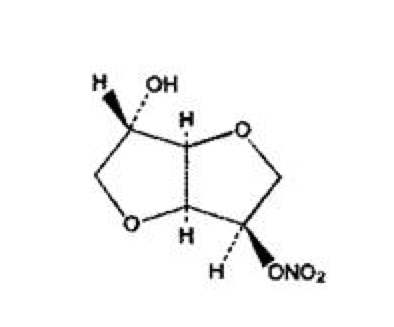
Isosorbide mononitrate
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Isosorbide mononitrate is an anti-anginal nitrate that is FDA approved for the {{{indicationType}}} of angina pectoris due to coronary artery disease.. Common adverse reactions include dizziness and headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Prophylaxis of Angina Pectoris
- Dosing Information
- The recommended starting dose is 30 mg (given as a single 30 mg tablet or as 1/2 of a 60 mg tablet) or 60 mg (given as a single tablet) once daily.
- After several days, the dosage may be increased to 120 mg (given as a single 120 mg tablet or as two 60 mg tablets) once daily. Rarely, 240 mg may be required.
- The daily dose of IMDUR Tablets should be taken in the morning on arising.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Isosorbide mononitrate in adult patients.
Non–Guideline-Supported Use
Prophylaxis of Rebleeding of Esophageal Varices
- Dosing Information
- 10 mg twice daily and titrated to 20 mg twice daily unless hypotension or headache occurred.[1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Isosorbide mononitrate in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Isosorbide mononitrate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Isosorbide mononitrate in pediatric patients.
Contraindications
- Hypersensitivity or idiosyncratic reactions to other nitrates or nitrites.
Warnings
- Amplification of the vasodilatory effects of IMDUR by sildenafil can result in severe hypotension. The time course and dose dependence of this interaction have not been studied. Appropriate supportive care has not been studied, but it seems reasonable to treat this as a nitrate overdose, with elevation of the extremities and with central volume expansion.
- The benefits of ISMN in patients with acute myocardial infarction or congestive heart failure have not been established; because the effects of isosorbide mononitrate are difficult to terminate rapidly, this drug is not recommended in these settings.
- If isosorbide mononitrate is used in these conditions, careful clinical or hemodynamic monitoring must be used to avoid the hazards of hypotension and tachycardia.
Precautions
- Severe hypotension, particularly with upright posture, may occur with even small doses of isosorbide mononitrate. This drug should, therefore, be used with caution in patients who may be volume depleted or who, for whatever reason, are already hypotensive. Hypotension induced by isosorbide mononitrate may be accompanied by paradoxical bradycardia and increased angina pectoris.
- Nitrate therapy may aggravate the angina caused by hypertrophic cardiomyopathy.
- In industrial workers who have had long-term exposure to unknown (presumably high) doses of organic nitrates, tolerance clearly occurs. Chest pain, acute myocardial infarction, and even sudden death have occurred during temporary withdrawal of nitrates from these workers, demonstrating the existence of true physical dependence. The importance of these observations to the routine, clinical use of oral isosorbide mononitrate is not known.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Isosorbide mononitrate in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Isosorbide mononitrate in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Isosorbide mononitrate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Isosorbide mononitrate during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Isosorbide mononitrate with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Isosorbide mononitrate with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Isosorbide mononitrate with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Isosorbide mononitrate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Isosorbide mononitrate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Isosorbide mononitrate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Isosorbide mononitrate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Isosorbide mononitrate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Isosorbide mononitrate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- IMDUR Extended Release Tablets should not be chewed or crushed and should be swallowed together with a half-glassful of fluid. Do not break the 30 mg tablet.
Monitoring
There is limited information regarding Monitoring of Isosorbide mononitrate in the drug label.
Condition1
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Isosorbide mononitrate in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Isosorbide mononitrate in the drug label.
Pharmacology
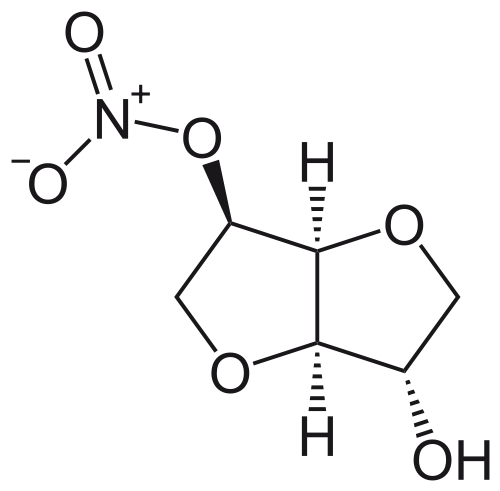
| |
Isosorbide mononitrate
| |
| Systematic (IUPAC) name | |
| 8-nitrooxy-2,6-dioxabicyclo[3.3.0]octan-4-ol | |
| Identifiers | |
| CAS number | |
| ATC code | C01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 191.139 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | >95% |
| Protein binding | <5% |
| Metabolism | Hepatic |
| Half life | 5 hours |
| Excretion | Renal: 93% |
| Therapeutic considerations | |
| Pregnancy cat. |
C (USA) |
| Legal status | |
| Routes | Oral |
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Isosorbide mononitrate in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Isosorbide mononitrate in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Isosorbide mononitrate in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Isosorbide mononitrate in the drug label.
Condition1
- Description
How Supplied
Storage
There is limited information regarding Isosorbide mononitrate Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Isosorbide mononitrate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Isosorbide mononitrate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Isosorbide mononitrate in the drug label.
Precautions with Alcohol
- Alcohol-Isosorbide mononitrate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- IMDUR®[2]
Look-Alike Drug Names
- A® — B®[3]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Merkel, C. (1996-12-21). "Randomised trial of nadolol alone or with isosorbide mononitrate for primary prophylaxis of variceal bleeding in cirrhosis. Gruppo-Triveneto per L'ipertensione portale (GTIP)". Lancet. 348 (9043): 1677–1681. ISSN 0140-6736. PMID 8973428. Unknown parameter
|coauthors=ignored (help) - ↑ "IMDUR (isosorbide mononitrate) tablet, extended release".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Isosorbide mononitrate |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Isosorbide mononitrate |Label Name=Isosorbide mononitrate11.png
}}
{{#subobject:
|Label Page=Isosorbide mononitrate |Label Name=Isosorbide mononitrate11.png
}}