Human parainfluenza viruses
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Human parainfluenza viruses (HPIVs) are a group of four distinct serotypes of single-stranded RNA viruses belonging to the paramyxovirus family. They are the second most common cause of lower respiratory tract infection in younger children. Repeated infection throughout the life of the host is not uncommon. Symptoms of later breakouts include upper respiratory tract illness as in a cold and sore throat. The incubation period of all four serotypes is 1 to 7 days. Parainfluenza viruses can be detected via cell culture, immunofluorescent microscopy, and PCR. Though no vaccines currently exist, research into vaccines for HPIV-1, -2, and -3 is underway. Parainfluenza viruses last only a few hours in the environment and are inactivated by soap and water.
Clinical features
Human parainfluenza viruses (HPIVs) are second to respiratory syncytial virus (RSV) as a common cause of lower respiratory tract disease in young children. Similar to RSV, HPIVs can cause repeated infections throughout life, usually manifested by an upper respiratory tract illness (e.g., a cold and/or sore throat). HPIVs can also cause serious lower respiratory tract disease with repeat infection (e.g., pneumonia, bronchitis, and bronchiolitis), especially among the elderly, and among patients with compromised immune systems. Each of the four HPIVs has different clinical and epidemiologic features. The most distinctive clinical feature of HPIV-1 and HPIV-2 is croup (i.e., laryngotracheobronchitis); HPIV-1 is the leading cause of croup in children, whereas HPIV-2 is less frequently detected. Both HPIV-1 and -2 can cause other upper and lower respiratory tract illnesses. HPIV-3 is more often associated with bronchiolitis and pneumonia. HPIV-4 is infrequently detected, possibly because it is less likely to cause severe disease. The incubation period for HPIVs is generally from 1 to 7 days.
The viruses
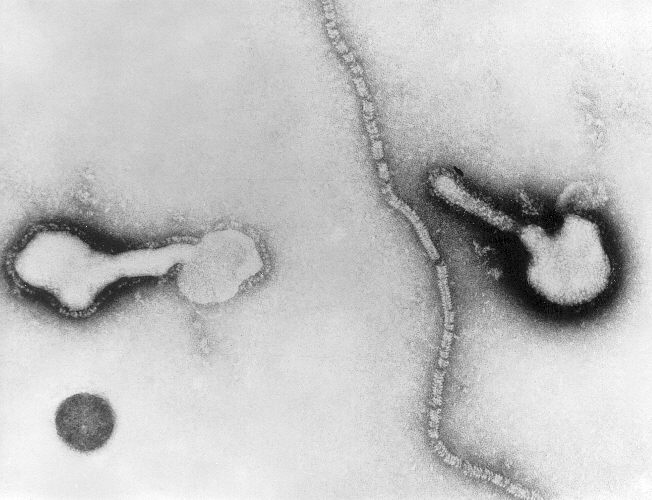
HPIVs are negative-sense, single-stranded RNA viruses that possess fusion and hemagglutinin-neuraminidase glycoprotein "spikes" on their surface. There are four serotypes types of HPIV (1 through 4) and two subtypes (4a and 4b). The virion varies in size (average diameter between 150 and 300 nm) and shape, is unstable in the environment (surviving a few hours on environmental surfaces), and is readily inactivated with soap and water.
Types
The four serotypes include:
- Human parainfluenza virus type 1: HPIV-1 (most common cause of croup; also other upper and lower respiratory tract illnesses typical)
- Human parainfluenza virus type 2: HPIV-2 (causes croup and other upper and lower respiratory tract illnesses)
- Human parainfluenza virus type 3: HPIV-3 (associated with bronchiolitis and pneumonia)
- Human parainfluenza virus type 4: HPIV-4 (includes subtypes 4a and 4b)
Epidemiologic features
HPIVs are spread from respiratory secretions through close contact with infected persons or contact with contaminated surfaces or objects. Infection can occur when infectious material contacts mucous membranes of the eyes, mouth, or nose, and possibly through the inhalation of droplets generated by a sneeze or cough. HPIVs can remain infectious in aerosols for over an hour. HPIVs are ubiquitous and infect most people during childhood. The highest rates of serious HPIV illnesses occur among young children. Serologic surveys have shown that 90% to 100% of children aged 5 years and older have antibodies to HPIV- 3, and about 75% have antibodies to HPIV-1 and -2. The different HPIV serotypes differ in their clinical features and seasonality. HPIV-1 causes biennial outbreaks of croup in the fall (presently in the United States during odd numbered years). HPIV-2 causes annual or biennial fall outbreaks. HPIV-3 peak activity occurs during the spring and early summer months each year, but the virus can be isolated throughout the year.
Diagnosis
Infection with HPIVs can be confirmed in two ways: 1) by isolation and identification of the virus in cell culture or by direct detection of the virus in respiratory secretions (usually, collected within one week of onset of symptoms) using immunofluorescence, enzyme immunoassay, or polymerase chin reaction assay, and 2) by demonstration of a significant rise in specific IgG antibodies between appropriately collected paired serum specimens or specific IgM antibodies in a single serum specimen.
Prevention
No vaccine is currently available to protect against infection caused by any of the HPIVs; however, researchers are developing vaccines against HPIV-1 and -3 infections. Passively acquired maternal antibodies may play a role in protection from HPIV types 1 and 2 in the first few months of life, highlighting the importance of breast-feeding. Strict attention to infection-control practices should decrease or prevent spread of infection. Frequent handwashing and not sharing items such as cups, glasses, and utensils with an infected person should decrease the spread of virus to others. Excluding children with colds or other respiratory illnesses (without fever) who are well enough to attend child care or school settings will probably not decrease the spread of HPIVs, because the viruses are often spread in the early stages of illness. In a hospital setting, spread of HPIVs can and should be prevented by strict attention to contact precautions, such as handwashing and wearing of protective gowns and gloves.
References
- American Academy of Pediatrics. Parainfluenza Viral Infections. In: Peter G, ed. 1997 Red Book: Report of the Committee on Infectious Diseases. 24th ed. Elk Grove Village, IL: American Academy of Pediatrics; 1997: 379.
- Collins PL, Chanock RM, McIntosh K. Parainfluenza viruses. In: Fields BN, Knipe DM, Howley PM, eds. Fields Virology. 3rd ed. Philadelphia: Lippincott-Raven; 1995: 1205-41.
- Glezen WP, Denny FW. Parainfluenza Viruses In: Evans A, Kaslow R, eds. Viral Infections in Humans: epidemiology and control. 4th ed. New York: Plenum; 1997:551-67.