Human Immunodeficiency Virus (HIV): Difference between revisions
No edit summary |
|||
| Line 32: | Line 32: | ||
==Classification== | ==Classification== | ||
==Early history== | ==Early history== | ||
Revision as of 21:03, 23 April 2012
Template:DiseaseDisorder infobox
| Human immunodeficiency virus | ||||||
|---|---|---|---|---|---|---|
 Stylized rendering of a cross section
of the human immunodeficiency virus | ||||||
| Virus classification | ||||||
| ||||||
| Species | ||||||
|
To read more about AIDS, click here.
To read about the difference between HIV & AIDS, click here.
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Template:HIV
Overview
Origin and discovery
- See AIDS origin
Classification
Early history
- See History of known cases and spread for early cases of HIV / AIDS
Transmission
Structure and genome
Tropism
Replication cycle
Genetic variability
The clinical course of infection
- For more details on this topic, see AIDS Diagnosis, AIDS Symptoms and Complications and WHO Disease Staging System for HIV Infection and Disease
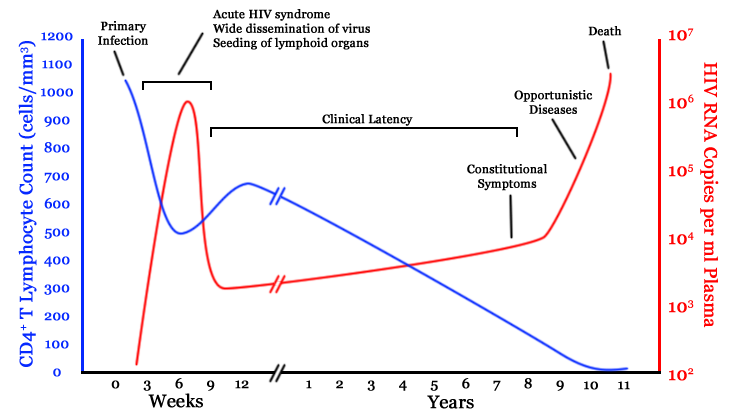
Infection with HIV-1 is associated with a progressive decrease of the CD4+ T cell count and an increase in viral load. The stage of infection can be determined by measuring the patient's CD4+ T cell count, and the level of HIV in the blood.
The initial infection with HIV generally occurs after transfer of body fluids from an infected person to an uninfected one. The first stage of infection, the primary, or acute infection, is a period of rapid viral replication that immediately follows the individual's exposure to HIV leading to an abundance of virus in the peripheral blood with levels of HIV commonly approaching several million viruses per mL.[1] This response is accompanied by a marked drop in the numbers of circulating CD4+ T cells. This acute viremia is associated in virtually all patients with the activation of CD8+ T cells, which kill HIV-infected cells, and subsequently with antibody production, or seroconversion. The CD8+ T cell response is thought to be important in controlling virus levels, which peak and then decline, as the CD4+ T cell counts rebound to around 800 cells per mL (the normal value is 1200 cells per mL ). A good CD8+ T cell response has been linked to slower disease progression and a better prognosis, though it does not eliminate the virus.[2] During this period (usually 2-4 weeks post-exposure) most individuals (80 to 90%) develop an influenza or mononucleosis-like illness called acute HIV infection, the most common symptoms of which may include fever, lymphadenopathy, pharyngitis, rash, myalgia, malaise, mouth and esophagal sores, and may also include, but less commonly, headache, nausea and vomiting, enlarged liver/spleen, weight loss, thrush, and neurological symptoms. Infected individuals may experience all, some, or none of these symptoms. The duration of symptoms varies, averaging 28 days and usually lasting at least a week.[3] Because of the nonspecific nature of these symptoms, they are often not recognized as signs of HIV infection. Even if patients go to their doctors or a hospital, they will often be misdiagnosed as having one of the more common infectious diseases with the same symptoms. Consequently, these primary symptoms are not used to diagnose HIV infection as they do not develop in all cases and because many are caused by other more common diseases. However, recognizing the syndrome can be important because the patient is much more infectious during this period. [4]
| sensitivity | specificity | |
|---|---|---|
| Fever | 88% | 50% |
| Malaise | 73% | 58% |
| Myalgia | 60% | 74% |
| Rash | 58% | 79% |
| Headache | 55% | 56% |
| Night sweats | 50% | 68% |
| Sore throat | 43% | 51% |
| Lymphadenopathy | 38% | 71% |
| Arthralgia | 28% | 87% |
| Nasal congestion | 18% | 62% |
A strong immune defense reduces the number of viral particles in the blood stream, marking the start of the infection's clinical latency stage. Clinical latency can vary between two weeks and 20 years. During this early phase of infection, HIV is active within lymphoid organs, where large amounts of virus become trapped in the follicular dendritic cells (FDC) network.[5] The surrounding tissues that are rich in CD4+ T cells may also become infected, and viral particles accumulate both in infected cells and as free virus. Individuals who are in this phase are still infectious. During this time, CD4+ CD45RO+ T cells carry most of the proviral load.[6]
When CD4+ T cell numbers decline below a critical level, cell-mediated immunity is lost, and infections with a variety of opportunistic microbes appear. The first symptoms often include moderate and unexplained weight loss, recurring respiratory tract infections (such as sinusitis, bronchitis, otitis media, pharyngitis), prostatitis, skin rashes, and oral ulcerations. Common opportunistic infections and tumors, most of which are normally controlled by robust CD4+ T cell-mediated immunity then start to affect the patient. Typically, resistance is lost early on to oral Candida species and to Mycobacterium tuberculosis, which leads to an increased susceptibility to oral candidiasis (thrush) and tuberculosis. Later, reactivation of latent herpes viruses may cause worsening recurrences of herpes simplex eruptions, shingles, Epstein-Barr virus-induced B-cell lymphomas, or Kaposi's sarcoma, a tumor of endothelial cells that occurs when HIV proteins such as Tat interact with Human Herpesvirus-8. Pneumonia caused by the fungus Pneumocystis jirovecii is common and often fatal. In the final stages of AIDS, infection with cytomegalovirus (another herpes virus) or Mycobacterium avium complex is more prominent. Not all patients with AIDS get all these infections or tumors, and there are other tumors and infections that are less prominent but still significant.
HIV test
Treatment
Epidemiology
AIDS denialism
A small minority of scientists and activists question the connection between HIV and AIDS,[7] the existence of HIV itself,[8] or the validity of current testing and treatment methods. These claims have been examined and widely rejected by the scientific community,[9] although they have had a political impact, particularly in South Africa, where governmental acceptance of AIDS denialism has been blamed for an ineffective response to that country's AIDS epidemic.[10][11][12]
Related Chapters
- AIDS
- HIV disease
- Hepatitis C with HIV coinfection
- Hepatitis B with HIV coinfection
- Tuberculosis and HIV coinfection
- HIV and tuberculosis coinfection : drug interaction
References
- ↑ Piatak, M., Jr, Saag, M. S., Yang, L. C., Clark, S. J., Kappes, J. C., Luk, K. C., Hahn, B. H., Shaw, G. M. and Lifson, J.D. (1993). "High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR". Science. 259 (5102): 1749–1754. doi:10.1126/science.8096089. PMID 8096089.
- ↑ Pantaleo G, Demarest JF, Schacker T, Vaccarezza M, Cohen OJ, Daucher M, Graziosi C, Schnittman SS, Quinn TC, Shaw GM, Perrin L, Tambussi G, Lazzarin A, Sekaly RP, Soudeyns H, Corey L, Fauci AS. (1997). "The qualitative nature of the primary immune response to HIV infection is a prognosticator of disease progression independent of the initial level of plasma viremia". Proc Natl Acad Sci U S A. 94 (1): 254–258. PMID 8990195.
- ↑ Kahn, J. O. and Walker, B. D. (1998). "Acute Human Immunodeficiency Virus type 1 infection". N. Engl. J. Med. 331 (1): 33–39. PMID 9647878.
- ↑ 4.0 4.1 Daar ES, Little S, Pitt J; et al. (2001). "Diagnosis of primary HIV-1 infection. Los Angeles County Primary HIV Infection Recruitment Network". Ann. Intern. Med. 134 (1): 25–9. PMID 11187417.
- ↑ Burton GF, Keele BF, Estes JD, Thacker TC, Gartner S. (2002). "Follicular dendritic cell contributions to HIV pathogenesis". Semin Immunol. 14 (4): 275–284. PMID 12163303.
- ↑ Clapham PR, McKnight A. (2001). "HIV-1 receptors and cell tropism". Br Med Bull. 58 (4): 43–59. PMID 11714623.
- ↑ Duesberg, P. H. (1988). "HIV is not the cause of AIDS". Science. 241 (4865): 514, 517. doi:10.1126/science.3399880. PMID 3399880.
- ↑ Papadopulos-Eleopulos, E., Turner, V. F., Papadimitriou, J., Page, B., Causer, D., Alfonso, H., Mhlongo, S., Miller, T., Maniotis, A. and Fiala, C. (2004). "A critique of the Montagnier evidence for the HIV/AIDS hypothesis". Med Hypotheses. 63 (4): 597&ndash, 601. PMID 15325002.
- ↑ For evidence of the scientific consensus that HIV is the cause of AIDS, see (for example):
- "The Durban Declaration". Nature. 406 (6791): 15–6. 2000. doi:10.1038/35017662. PMID 10894520. - full text here.
- Cohen, J. (1994). "The Controversy over HIV and AIDS" (PDF). Science. 266 (5191): 1642&ndash, 1649.
- Various. "Focus on the HIV-AIDS Connection: Resource links". National Institute of Allergy and Infectious Diseases. Retrieved 2006-09-07.
- O'Brien SJ, Goedert JJ (1996). "HIV causes AIDS: Koch's postulates fulfilled". Curr. Opin. Immunol. 8 (5): 613–8. PMID 8902385.
- Galéa P, Chermann JC (1998). "HIV as the cause of AIDS and associated diseases". Genetica. 104 (2): 133–42. PMID 10220906.
- ↑ Watson J (2006). "Scientists, activists sue South Africa's AIDS 'denialists'". Nat. Med. 12 (1): 6. doi:10.1038/nm0106-6a. PMID 16397537.
- ↑ Baleta A (2003). "S Africa's AIDS activists accuse government of murder". Lancet. 361 (9363): 1105. doi:10.1016/S0140-6736(03)12909-1. PMID 12672319.
- ↑ Cohen J (2000). "South Africa's new enemy". Science. 288 (5474): 2168–70. doi:10.1126/science.288.5474.2168. PMID 10896606.
External links
- UNAIDS - Joint United Nations Programme on HIV/AIDS webpage
- PlusNews, The United Nations HIV and AIDS news service
- History of AIDS research at the NIH
- Medecins Sans Frontieres/Doctors Without Borders HIV/AIDS Pages
- AIDSinfo - HIV/AIDS Information - Comprehensive resource for HIV/AIDS treatment and clinical trial information from the U. S. Department of Health and Human Services
- HIV InSite - University of California San Francisco
- AIDS.ORG: Educating - Raising HIV Awareness - Building Community
- AIDSPortal - Latest policy, research, guidelines and case studies
- AIDS.gov Portal for all Federal domestic HIV/AIDS information and resources
- News media
- "The Age of Aids" March 30, 2006 Frontline
- Everything you wanted to know about HIV and AIDS — Provided by New Scientist.
- "The Graying of AIDS" - Older Americans living with HIV face unique challenges - TIME magazine, 08/14/06
- "Elite" HIV patients mystify doctors - Aug 16, 2006 | Reuters on Topix.net
- Online textbooks
- HIV Medicine 2006, medical textbook, 14th edition, 825 pages (free download)
- "The Molecules of HIV" information resource
- Advocacy
- Be the Generation - Information on HIV Vaccine Clinical Research in 20 American Cities
- Media Campaign: HIV leads to AIDS
- FightAIDS@Home
- HIV-AIDS-Counseling-Online.com
- Articles
- The Mechanism of HIV-1 Core Assembly: Insights from Three-Dimensional Reconstructions of Authentic Virions
- Unsafe Health Care and the HIV/AIDS Pandemic 2003
- The role of dendritic cells in HIV pathogenesis
- The HIV databases, Los Alamos National Laboratory
- Multimedia/video
- HIV DERMATOLOGY
- How Aids Works (with animation)
- Watch an animated tutorial on the life cycle of HIV
- Video of Treatment Update 2006 from the Conference on Retrovirus'
- Other sites
- HIV/AIDS at About.com provides a comprehensive collection of HIV and AIDS information, articles, statistics, and forums.
- POZ provides information and networking opportunities for the HIV community.
- AIDSmeds is a place to find complete and easy-to-read information on treating HIV & AIDS.
- AEGiS.org: AIDS Education Global Information System- Patient/clinician information & Historical news and treatment database
- AIDS Community Research Initiative of America - Community-based research and education for people living with HIV
- Data about people living with HIV/AIDS in the world (regional totals and percentage of adults with AIDS that are women)- from Data360
- Resources for HIV/AIDS and Sexual and Reproductive Health Integration Johns Hopkins Bloomberg School of Public Health, Center for Communication Programs