Halothane
 | |
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Pharmacokinetic data | |
| Metabolism | Hepatic (CYP2E1[1]) |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
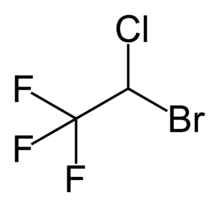
| Formula | C2HBrClF3 |
| Molar mass | 197.381 g/mol |
|
WikiDoc Resources for Halothane |
|
Articles |
|---|
|
Most recent articles on Halothane |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Halothane at Clinical Trials.gov Clinical Trials on Halothane at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Halothane
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Halothane Discussion groups on Halothane Directions to Hospitals Treating Halothane Risk calculators and risk factors for Halothane
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Halothane |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Halothane vapour (or Fluothane) is an inhalational general anaesthetic. Its systematic name is 2-Bromo-2-chloro-1,1,1-trifluoroethane. It is the only inhalational anaesthetic agent containing a bromine atom. It is colourless and pleasant-smelling, but unstable in light. It is packaged in dark-coloured bottles and contains 0.01% thymol as a stabilising agent.
Adverse effects
All volatile anaesthetics such as halothane can trigger malignant hyperthermia in genetically susceptible individuals. The caffeine-halothane contracture test was developed to directly test muscle biopsy specimens for this susceptibility. This test may be replaced by genetic testing in the future.
Related substances
Chemically, halothane is not an ether. Attempts to find anaesthetics with less metabolism led to halogenated ethers such enflurane and isoflurane. The incidence of hepatic reactions with these agents is less. The degree of hepatotoxic potential of enflurane is controversial, although it is minimally metabolised. Isoflurane is essentially not metabolised and reports of associated liver injury are quite rare.
Physical properties
| Boiling point: | 50.2 °C | (at 101.325 kPa) |
| Density: | 1.868 g/cm³ | (at 20 °C) |
| Molecular Weight: | 197.4 u | |
| Vapor pressure: | 244 mmHg | (at 20 °C) |
| 288 mmHg | (at 24 °C) | |
| MAC: | 0.75 | vol % |
| Blood:Gas Partition coefficient: | 2.5 | |
| Oil:Gas Partition coefficient: | 224 |
History
This halogenated hydrocarbon was first synthesised by C. W. Suckling of Imperial Chemical Industries (ICI) in 1951 and was first used clinically by M. Johnstone in Manchester in 1956. Halothane became popular as a nonflammable general anaesthetic replacing other volatile anaesthetics such as diethyl ether and cyclopropane. Use of the anesthetic was phased out during the 1980s and 1990s as newer anesthetic agents became popular. Halothane retains some use in veterinary surgery and in the Third World because of its lower cost.
Halothane was given to many millions of adult and pediatric patients worldwide from its introduction in 1956 through the 1980s. Its properties include cardiac depression at high levels, cardiac sensitisation to catecholamines such as norepinephrine, and potent bronchial relaxation. Its lack of airway irritation made it a common inhalation induction agent in pediatric anaesthesia. Due to its cardiac depressive effect, it was contraindicated in patients with cardiac failure. Halothane was also contraindicated in patients susceptible to cardiac arrythmias, or in situations related to high catecholamine levels such as pheochromocytoma.
Repeated exposure to halothane in adults was noted in rare cases to result in severe liver injury. This occurred in about 1 in 35,000 exposures. The resulting syndrome was referred to as halothane hepatitis, and is thought to result from the metabolism of halothane to trifluoroacetic acid via oxidative reactions in the liver. About 20% of inhaled halothane is metabolised by the liver and these products are excreted in the urine. The hepatitis syndrome had a mortality rate of 30% to 70%. Concern for hepatitis resulted in a dramatic reduction in the use of halothane for adults. It was replaced in the 1980s by enflurane and isoflurane. By the year 2005 the common volatile anaesthetics in use were isoflurane, sevoflurane, and desflurane. Since the risk of halothane hepatitis in children was substantially lower than in adults, halothane saw continued use in pediatrics in the 1990s. However, by the year 2000 sevoflurane had largely replaced the use of halothane in children.
References
- Atkinson, Rushman, Lee. A Synopsis of Anaesthesia. 1987.
- Eger, Eisenkraft, Weiskopf. The Pharmacology of Inhaled Anesthetics. 2003.
External links
Template:SIB Template:General anesthetics
- Pages using duplicate arguments in template calls
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Anesthetics
- Hepatitis
- Organohalides
- Drugs