Guanidine
| Template:Chembox header| Guanidine | |
|---|---|
| |
| Chemical name | Guanidine |
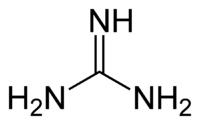
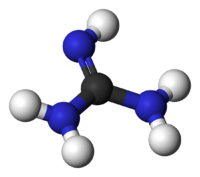
| Chemical formula | CH5N3 |
| Molecular mass | 59.0706 g mol−1 |
| CAS number | [113-00-8] |
| Density | x.xxx g cm−3 |
| Melting point | 50 °C |
| Boiling point | xx.x °C |
| SMILES | C(=N)(N)N |
| Template:Chembox header | Disclaimer and references | |
Overview
Guanidine is a crystalline compound of strong alkalinity formed by the oxidation of guanine. It is used in the manufacture of plastics and explosives. It is found in urine as a normal product of protein metabolism.
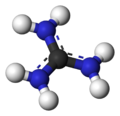
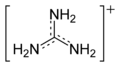
Guanidinium cation
With a pKa of 12.5, guanidine is protonated in physiological conditions, with a charge of +1. This conjugate acid of guanidine is called the guanidinium cation, [CH6N3]+.
-
canonical forms
Notable guanidinium salts include guanidine hydrochloride, which has chaotropic properties and is used to denature proteins. Empirically, guanidine hydrochloride is known to denature proteins with a linear relationship between concentration and free energy of unfolding.
Guanidine derivatives
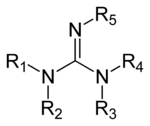
Guanidines are a group of organic compounds sharing a common functional group with the general structure (R1R2N)(R3R4N)C=N-R5. The central bond within this group is that of an imine; the other recognizable motif within this group is an aminal. Examples of guanidines are triazabicyclodecene and saxitoxin.
Use as an alternative fuel
Guanidine is currently being considered as an alternative fuel. In the presence of a catalyst, a mole of free-base guanidine combines with 2 moles of water to form 3 moles of ammonia and 1 mole of carbon dioxide. The ammonia can be used directly as a fuel for internal combustion engines, or decomposed into nitrogen and hydrogen gas for use in fuel cells. The guanidine could be supplied as a fuel in solid form as pure guanidine (melting point ~ 50 C) or as a lower melting point eutectic mixture with urea. Guanidine could also be supplied as solutions in ethanol, as a replacement for the gasoline component in E85 fuel.