Granisetron (tablet): Difference between revisions
Shanshan Cen (talk | contribs) No edit summary |
m (Protected "Granisetron (tablet)": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (29 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag={{SC}} | |authorTag={{SC}} | ||
|indicationType= | |genericName=Granisetron hydrochloride | ||
|blackBoxWarningTitle= | |aOrAn=an | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;"> | |drugClass=[[antinauseant]] and [[antiemetic]] agent | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of | |indicationType=prevention | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Granisetron | |indication=[[nausea]] and [[vomiting]] associated with:1) initial and repeat courses of emetogenic cancer therapy, including high-dose [[cisplatin]];2) [[radiation]], including total body irradiation and fractionated abdominal radiation | ||
|adverseReactions=[[asthenia]], [[headache]], [[somnolence]], and [[fever]] | |||
|blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | |||
|blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | |||
* Content | |||
<!--Adult Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Adult)--> | |||
|fdaLIADAdult=====Indications==== | |||
Granisetron hydrochloride tablets are indicated for the prevention of: | |||
*Nausea and vomiting associated with initial and repeat courses of emetogenic cancer therapy, including high-dose cisplatin. | |||
*Nausea and vomiting associated with radiation, including total body irradiation and fractionated abdominal radiation. | |||
====Dosage==== | |||
=====Emetogenic Chemotherapy===== | |||
The recommended adult dosage of oral granisetron hydrochloride tablets is 2 mg once daily or 1 mg twice daily. In the 2 mg once-daily regimen, two 1 mg tablets are given up to 1 hour before chemotherapy. In the 1 mg twice-daily regimen, the first 1 mg tablet is given up to 1 hour before chemotherapy, and the second tablet 12 hours after the first. Either regimen is administered only on the day(s) chemotherapy is given. Continued treatment, while not on chemotherapy, has not been found to be useful. | |||
=====Radiation===== | |||
The recommended adult dosage of oral granisetron hydrochloride tablets is 2 mg once daily. Two 1 mg tablets are taken within 1 hour of radiation. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
|fdaLIADPed=====Indications==== | |||
Granisetron hydrochloride tablets are indicated for the prevention of: | |||
*Nausea and vomiting associated with initial and repeat courses of emetogenic cancer therapy, including high-dose cisplatin. | |||
*Nausea and vomiting associated with radiation, including total body irradiation and fractionated abdominal radiation. | |||
====Dosage==== | |||
Safety and effectiveness in pediatric patients have not been established. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Granisetron (tablet) in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Granisetron (tablet) in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Granisetron (tablet) in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Granisetron (tablet) in pediatric patients. | ||
| | |contraindications=Granisetron hydrochloride tablets are contraindicated in patients with known hypersensitivity to the drug or any of its components. | ||
|warnings=====Serotonin Syndrome==== | |||
The development of [[serotonin syndrome]] has been reported with 5-HT3 receptor antagonists. Most reports have been associated with concomitant use of serotonergic drugs (e.g., [[selective serotonin reuptake inhibitors]] (SSRIs), [[serotonin and norepinephrine reuptake inhibitors]] (SNRIs), [[monoamine oxidase inhibitors]], mirtazapine, fentanyl, lithium, tramadol, and intravenous methylene blue). Some of the reported cases were fatal. Serotonin syndrome occurring with overdose of another 5-HT3 receptor antagonist alone has also been reported. The majority of reports of serotonin syndrome related to 5-HT3 receptor antagonist use occurred in a post-anesthesia care unit or an infusion center. | |||
Symptoms associated with serotonin syndrome may include the following combination of signs and symptoms: mental status changes (e.g., [[agitation]], [[hallucinations]], [[delirium]], and [[coma]]), autonomic instability (e.g., [[tachycardia]], labile blood pressure, [[dizziness]], [[diaphoresis]], [[flushing]], [[hyperthermia]]), neuromuscular symptoms (e.g., [[tremor]], [[rigidity]], [[myoclonus]], [[hyperreflexia]], [[incoordination]]), [[seizures]], with or without gastrointestinal symptoms (e.g., nausea, vomiting, [[diarrhea]]). Patients should be monitored for the emergence of serotonin syndrome, especially with concomitant use of granisetron and other serotonergic drugs. If symptoms of serotonin syndrome occur, discontinue granisetron and initiate supportive treatment. Patients should be informed of the increased risk of serotonin syndrome, especially if granisetron is used concomitantly with other serotonergic drugs | |||
|clinicalTrials======Chemotherapy-Induced Nausea and Vomiting===== | |||
In patients receiving granisetron hydrochloride tablets 1 mg twice a day for 1, 7 or 14 days, or 2 mg once a day for 1 day, adverse experiences reported in more than 5% of the patients with comparator and placebo incidences are listed in Table 4. | |||
[[File:granisetron tablet3.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
Other adverse events reported in clinical trials were: | |||
*Gastrointestinal: In single-day dosing studies in which adverse events were collected for 7 days, nausea (20%) and vomiting (12%) were recorded as adverse events after the 24-hour efficacy assessment period. | |||
*Hepatic: In comparative trials, elevation of [[AST]] and [[ALT]] (>2 times the upper limit of normal) following the administration of granisetron hydrochloride tablets occurred in 5% and 6% of patients, respectively. These frequencies were not significantly different from those seen with comparators (AST: 2%; ALT: 9%). | |||
*Cardiovascular: [[Hypertension]] (1%); [[hypotension]], [[angina pectoris]], [[atrial fibrillation]], and [[syncope]] have been observed rarely. | |||
*Central Nervous System: [[Dizziness]] (5%), [[insomnia]] (5%), [[anxiety]] (2%), [[somnolence]] (1%). One case compatible with, but not diagnostic of, [[extrapyramidal symptoms]] has been reported in a patient treated with granisetron hydrochloride tablets. | |||
*Hypersensitivity: Rare cases of hypersensitivity reactions, sometimes severe (eg, [[anaphylaxis]], [[shortness of breath]], hypotension, [[urticaria]]) have been reported. | |||
*Other: Fever (5%). Events often associated with chemotherapy also have been reported: [[leukopenia]] (9%), decreased appetite (6%), [[anemia]] (4%), [[alopecia]] (3%), [[thrombocytopenia]] (2%). | |||
Over 5000 patients have received injectable granisetron hydrochloride in clinical trials. | |||
Table 5 gives the comparative frequencies of the five commonly reported adverse events (≥ 3%) in patients receiving granisetron hydrochloride injection, 40 mcg/kg, in single-day chemotherapy trials. These patients received chemotherapy, primarily cisplatin, and intravenous fluids during the 24-hour period following granisetron hydrochloride injection administration. | |||
[[File:granisetron tablet4.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
In the absence of a placebo group, there is uncertainty as to how many of these events should be attributed to granisetron hydrochloride, except for headache, which was clearly more frequent than in comparison groups. | |||
=====Radiation-Induced Nausea and Vomiting===== | |||
In controlled clinical trials, the adverse events reported by patients receiving granisetron hydrochloride tablets and concurrent radiation were similar to those reported by patients receiving granisetron hydrochloride tablets prior to chemotherapy. The most frequently reported adverse events were diarrhea, [[asthenia]], and [[constipation]]. [[Headache]], however, was less prevalent in this patient population. | |||
|postmarketing=[[QT prolongation]] has been reported with granisetron hydrochloride. | |||
|drugInteractions=Granisetron does not induce or inhibit the cytochrome [[P450|P-450]] drug-metabolizing enzyme system in vitro. There have been no definitive drug-drug interaction studies to examine pharmacokinetic or pharmacodynamic interaction with other drugs; however, in humans, granisetron hydrochloride injection has been safely administered with drugs representing [[benzodiazepines]], [[neuroleptics]], and anti-ulcer medications commonly prescribed with [[antiemetic]] treatments. Granisetron hydrochloride injection also does not appear to interact with emetogenic cancer chemotherapies. Because granisetron is metabolized by hepatic cytochrome P-450 drug-metabolizing enzymes, inducers or inhibitors of these enzymes may change the clearance and, hence, the half-life of granisetron. No specific interaction studies have been conducted in anesthetized patients. In addition, the activity of the cytochrome P-450 subfamily 3A4 (involved in the metabolism of some of the main narcotic analgesic agents) is not modified by granisetron hydrochloride in vitro. | |||
In in vitro human microsomal studies, [[ketoconazole]] inhibited ring oxidation of granisetron hydrochloride. However, the clinical significance of in vivo pharmacokinetic interactions with ketoconazole is not known. In a human pharmacokinetic study, hepatic enzyme induction with [[phenobarbital]] resulted in a 25% increase in total plasma clearance of intravenous granisetron hydrochloride. The clinical significance of this change is not known. | |||
QT prolongation has been reported with granisetron hydrochloride. Use of granisetron hydrochloride in patients concurrently treated with drugs known to prolong the QT interval and/or are arrhythmogenic, this may result in clinical consequences. | |||
Serotonin syndrome (including altered mental status, autonomic instability, and neuromuscular symptoms) has been described following the concomitant use of 5-HT3 receptor antagonists and other serotonergic drugs, including selective serotonin reuptake inhibitors (SSRIs) and serotonin and noradrenaline reuptake inhibitors (SNRIs). | |||
|FDAPregCat=B | |||
|useInPregnancyFDA=Teratogenic Effect: | |||
Reproduction studies have been performed in pregnant rats at oral doses up to 125 mg/kg/day (750 mg/m2/day, 507 times the recommended human dose based on body surface area) and pregnant rabbits at oral doses up to 32 mg/kg/day (378 mg/m2/day, 255 times the recommended human dose based on body surface area) and have revealed no evidence of impaired fertility or harm to the fetus due to granisetron. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. | |||
|AUSPregCat=B1 | |||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing=It is not known whether granisetron is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when granisetron hydrochloride is administered to a nursing woman. | |||
|useInPed=Safety and effectiveness in pediatric patients have not been established. | |||
|useInGeri=*The ranges of the pharmacokinetic parameters in elderly volunteers (mean age 71 years), given a single 40 mcg/kg intravenous dose of granisetron hydrochloride injection, were generally similar to those in younger healthy volunteers; mean values were lower for clearance and longer for half-life in the elderly. | |||
*During clinical trials, 325 patients 65 years of age or older received granisetron hydrochloride tablets; 298 were 65 to 74 years of age, and 27 were 75 years of age or older. Efficacy and safety were maintained with increasing age. | |||
*For the prevention of nausea and vomiting of both metogenic chemotherapy and radiation, no dosage adjustment is recommended. | |||
|useInGender=The effects of gender on the pharmacokinetics of granisteron hydrochloride tablets have not been studied. However, after intravenous infusion of granisetron hydrochloride, no difference in mean AUC was found between males and females, although males had a higher Cmax generally. | |||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair=*Total clearance of granisetron was not affected in patients with severe renal failure who received a single 40 mcg/kg intravenous dose of granisetron hydrochloride injection. | |||
*For the prevention of nausea and vomiting of both metogenic chemotherapy, no dosage adjustment is recommended. | |||
|useInHepaticImpair=*A pharmacokinetic study with intravenous granisetron hydrochloride in patients with hepatic impairment due to neoplastic liver involvement showed that total clearance was approximately halved compared to patients without hepatic impairment. Given the wide variability in pharmacokinetic parameters noted in patients, dosage adjustment in patients with hepatic functional impairment is not necessary. | |||
*For the prevention of nausea and vomiting of both metogenic chemotherapy, no dosage adjustment is recommended. | |||
|administration=* Tablets for oral administration | |||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
|overdose=There is no specific treatment for granisetron hydrochloride overdosage. In case of overdosage, symptomatic treatment should be given. Overdosage of up to 38.5 mg of granisetron hydrochloride injection has been reported without symptoms or only the occurrence of a slight headache. | |||
|drugBox={{drugbox2 | |||
| Verifiedfields = changed | |||
| verifiedrevid = 461123553 | |||
| IUPAC_name = 1-methyl-''N''-((1''R'',3''r'',5''S'')-9-methyl-9-azabicyclo[3.3.1]nonan-3-yl)-1''H''-indazole-3-carboxamide | |||
| image = granisetron.png | |||
| width = 185 | |||
| image2 = Granisetron 3D.png | |||
<!--Clinical data--> | |||
| tradename = Kytril | |||
| Drugs.com = {{drugs.com|monograph|granisetron-hydrochloride}} | |||
| MedlinePlus = a601211 | |||
| pregnancy_category = | |||
| pregnancy_AU = B1 | |||
| legal_status = | |||
| legal_AU = S4 | |||
| legal_US = Rx-only | |||
| legal_UK = POM | |||
| routes_of_administration = Oral, intravenous, transdermal | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = 60% | |||
| protein_bound = 65% | |||
| metabolism = Hepatic | |||
| elimination_half-life = 3–14 hours | |||
| excretion = Renal 11–12%, faecal 38% | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 109889-09-0 | |||
| ATC_prefix = A04 | |||
| ATC_suffix = AA02 | |||
| PubChem = 3510 | |||
| IUPHAR_ligand2 = 2292 | |||
| IUPHAR_ligand = 2300 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00889 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 10482033 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = WZG3J2MCOL | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D04370 | |||
| ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| ChEBI = 5537 | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 519643 | |||
<!--Chemical data--> | |||
| C=18 | H=24 | N=4 | O=1 | |||
| molecular_weight = 312.41 g/mol | |||
| smiles = CN4[C@@H]1CCC[C@H]4C[C@H](C1)NC(=O)c3nn(C)c2ccccc23 | |||
| InChI = 1/C18H24N4O/c1-21-13-6-5-7-14(21)11-12(10-13)19-18(23)17-15-8-3-4-9-16(15)22(2)20-17/h3-4,8-9,12-14H,5-7,10-11H2,1-2H3,(H,19,23)/t12-,13+,14- | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C18H24N4O/c1-21-13-6-5-7-14(21)11-12(10-13)19-18(23)17-15-8-3-4-9-16(15)22(2)20-17/h3-4,8-9,12-14H,5-7,10-11H2,1-2H3,(H,19,23)/t12-,13+,14- | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = MFWNKCLOYSRHCJ-BTTYYORXSA-N | |||
}} | }} | ||
<!--Structure--> | |||
|structure=Granisetron hydrochloride tablets contain granisetron hydrochloride, an antinauseant and antiemetic agent. Chemically it is endo-N-(9-methyl-9-azabicyclo [3.3.1] non-3-yl)-1-methyl-1H-indazole-3-carboxamide hydrochloride with a molecular weight of 348.9 (312.4 free base). Its empirical formula is C18H24N4O•HCl while its chemical structure is: | |||
[[File:granisetron tablet5.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|PD=Granisetron is a selective 5-hydroxytryptamine3 (5-HT3) receptor antagonist with little or no affinity for other serotonin receptors, including 5-HT1; 5-HT1A; 5-HT1B/C; 5-HT2; for alpha1-, alpha2-, or beta-adrenoreceptors; for dopamine-D2; or for histamine-H1; benzodiazepine; picrotoxin or opioid receptors. | |||
Serotonin receptors of the 5-HT3 type are located peripherally on vagal nerve terminals and centrally in the chemoreceptor trigger zone of the area postrema. During chemotherapy that induces vomiting, mucosal enterochromaffin cells release serotonin, which stimulates 5-HT3 receptors. This evokes vagal afferent discharge, inducing vomiting. Animal studies demonstrate that, in binding to 5-HT3 receptors, granisetron blocks serotonin stimulation and subsequent vomiting after emetogenic stimuli such as cisplatin. In the ferret animal model, a single granisetron injection prevented vomiting due to high-dose cisplatin or arrested vomiting within 5 to 30 seconds. | |||
In most human studies, granisetron has had little effect on blood pressure, heart rate or ECG. No evidence of an effect on plasma prolactin or aldosterone concentrations has been found in other studies. | |||
Following single and multiple oral doses, granisetron hydrochloride tablets slowed colonic transit in normal volunteers. However, granisetron hydrochloride had no effect on oro-cecal transit time in normal volunteers when given as a single intravenous (IV) infusion of 50 mcg/kg or 200 mcg/kg. | |||
|PK=In healthy volunteers and adult cancer patients undergoing chemotherapy, administration of granisetron hydrochloride tablets produced mean pharmacokinetic data shown in Table 1. | |||
[[File:granisetron tablet6.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
*Absorption: When granisetron hydrochloride tablets were administered with food, AUC was decreased by 5% and Cmax increased by 30% in non-fasted healthy volunteers who received a single dose of 10 mg. | |||
*Distribution: Plasma protein binding is approximately 65% and granisetron distributes freely between plasma and red blood cells. | |||
*Metabolism: Granisetron metabolism involves N-demethylation and aromatic ring oxidation followed by conjugation. In vitro liver microsomal studies show that granisetron's major route of metabolism is inhibited by ketoconazole, suggestive of metabolism mediated by the cytochrome P-450 3A subfamily. Animal studies suggest that some of the metabolites may also have 5-HT3 receptor antagonist activity. | |||
*Elimination: Clearance is predominantly by hepatic metabolism. In normal volunteers, approximately 11% of the orally administered dose is eliminated unchanged in the urine in 48 hours. The remainder of the dose is excreted as metabolites, 48% in the urine and 38% in the feces. | |||
|nonClinToxic=There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | |||
<!--Clinical Studies--> | |||
|clinicalStudies=====Chemotherapy-Induced Nausea and Vomiting==== | |||
Granisetron hydrochloride tablets prevent nausea and vomiting associated with initial and repeat courses of emetogenic cancer therapy, as shown by 24-hour efficacy data from studies using both moderately-and highly-emetogenic chemotherapy. | |||
''Moderately Emetogenic Chemotherapy'': The first trial compared granisteron hydrochloride tablet doses of 0.25 mg to 2 mg twice a day, in 930 cancer patients receiving, principally, cyclophosphamide, carboplatin, and cisplatin (20 mg/m2 to 50 mg/m2). Efficacy was based on complete response (ie, no vomiting, no moderate or severe nausea, no rescue medication), no vomiting, and no nausea. Table 2 summarizes the results of this study. | |||
[[File:granisetron tablet7.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
Results from a second double-blind, randomized trial evaluating granisetron hydrochloride tablets 2 mg once a day and granisetron hydrochloride tablets 1 mg twice a day were compared to prochlorperazine 10 mg twice a day derived from a historical control. At 24 hours, there was no statistically significant difference in efficacy between the two granisetron hydrochloride tablet regimens. Both regimens were statistically superior to the prochlorperazine control regimen (see Table 3). | |||
[[File:granisetron tablet8.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
Results from a granisetron hydrochloride tablets 2 mg once a day alone treatment arm in a third double-blind, randomized trial, were compared to prochlorperazine (PCPZ), 10 mg twice a day, derived from a historical control. The 24-hour results for granisetron hydrochloride tablets 2 mg once a day were statistically superior to PCPZ for all efficacy parameters: complete response (58%), no vomiting (79%), no nausea (51%), total control (49%). The PCPZ rates are shown in Table 3. | |||
''Cisplatin-Based Chemotherapy'': The first double-blind trial compared granisetron hydrochloride tablets 1 mg twice a day, relative to placebo (historical control), in 119 cancer patients receiving high-dose cisplatin (mean dose 80 mg/m2). At 24 hours, granisetron hydrochloride tablets 1 mg twice a day was significantly (P <0.001) superior to placebo (historical control) in all efficacy parameters: complete response (52%), no vomiting (56%) and no nausea (45%). The placebo rates were 7%, 14%, and 7%, respectively, for the three efficacy parameters. | |||
Results from a granisetron hydrochloride tablets 2 mg once a day alone treatment arm in a second double-blind, randomized trial, were compared to both granisetron hydrochloride tablets 1 mg twice a day and placebo historical controls. The 24-hour results for granisetron hydrochloride tablets 2 mg once a day were: complete response (44%), no vomiting (58%), no nausea (46%), total control (40%). The efficacy of granisetron hydrochloride tablets 2 mg once a day was comparable to granisetron hydrochloride tablets 1 mg twice a day and statistically superior to placebo. The placebo rates were 7%, 14%, 7%, and 7%, respectively, for the four parameters. | |||
No controlled study comparing granisetron injection with the oral formulation to prevent chemotherapy-induced nausea and vomiting has been performed. | |||
====Radiation-Induced Nausea and Vomiting==== | |||
''Total Body Irradiation'': In a double-blind randomized study, 18 patients receiving granisetron hydrochloride tablets, 2 mg daily, experienced significantly greater antiemetic protection compared to patients in a historical negative control group who received conventional (non-5-HT3 antagonist) antiemetics. Total body irradiation consisted of 11 fractions of 120 cGy administered over 4 days, with three fractions on each of the first 3 days, and two fractions on the fourth day. Granisetron hydrochloride tablets were given one hour before the first radiation fraction of each day. | |||
Twenty-two percent (22%) of patients treated with granisetron hydrochloride tablets did not experience vomiting or receive rescue antiemetics over the entire 4-day dosing period, compared to 0% of patients in the historical negative control group (P <0.01). | |||
In addition, patients who received granisetron hydrochloride tablets also experienced significantly fewer emetic episodes during the first day of radiation and over the 4-day treatment period, compared to patients in the historical negative control group. The median time to the first emetic episode was 36 hours for patients who received granisetron hydrochloride tablets. | |||
''Fractionated Abdominal Radiation'': The efficacy of granisetron hydrochloride tablets, 2 mg daily, was evaluated in a double-blind, placebo-controlled randomized trial of 260 patients. Granisetron hydrochloride tablets were given 1 hour before radiation, composed of up to 20 daily fractions of 180 to 300 cGy each. The exceptions were patients with seminoma or those receiving whole abdomen irradiation who initially received 150 cGy per fraction. Radiation was administered to the upper abdomen with a field size of at least 100 cm2. | |||
The proportion of patients without emesis and those without nausea for granisetron hydrochloride tablets, compared to placebo, was statistically significant (P <0.0001) at 24 hours after radiation, irrespective of the radiation dose. Granisetron hydrochloride was superior to placebo in patients receiving up to 10 daily fractions of radiation, but was not superior to placebo in patients receiving 20 fractions. | |||
Patients treated with granisetron hydrochloride tablets (n=134) had a significantly longer time to the first episode of vomiting (35 days vs. 9 days, P <0.001) relative to those patients who received placebo (n=126), and a significantly longer time to the first episode of nausea (11 days vs. 1 day, P <0.001). Granisetron hydrochloride provided significantly greater protection from nausea and vomiting than placebo. | |||
|howSupplied=Granisetron hydrochloride tablets 1 mg are white round film coated tablets debossed cor over 198 on one side and plain on the other side. | |||
They are supplied as follows: | |||
Blister cards of 2 unit of use tablets: NDC 64720-198-98 | |||
Blister cards of 20 unit of use tablets: NDC 64720-198-97 (intended for institutional use only) | |||
Bottles of 20: NDC 64720-198-02 (intended for institutional use only) | |||
Bottles of 100: NDC 64720-198-10 (intended for institutional use only) | |||
|storage=Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. | |||
Keep container closed tightly. Protect from light. | |||
Keep this and all drugs out of the reach of children. | |||
|packLabel=[[File:granisetron tablet9.jpeg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
[[File:granisetron tablet10.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|fdaPatientInfo=Advise patients of the possibility of serotonin syndrome with concomitant use of granisetron and another serotonergic agent such as medications to treat depression and migraines. Advise patients to seek immediate medical attention if the following symptoms occur: changes in mental status, autonomic instability, neuromuscular symptoms with or without gastrointestinal symptoms. | |||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | |||
|brandNames=* GRANISETRON HYDROCHLORIDE®<ref>{{Cite web | title =GRANISETRON HYDROCHLORIDE- granisetron hydrochloride tablet, film coated | url =http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d0e05816-ccf8-4008-b5de-4d3fbc3de3c1 }}</ref> | |||
|drugShortage= | |||
}} | |||
<!--Pill Image--> | |||
<!--Label Display Image--> | |||
<!--Category--> | |||
[[Category:Drug]] | |||
Latest revision as of 16:26, 20 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Shanshan Cen, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Granisetron (tablet) is an antinauseant and antiemetic agent that is FDA approved for the prevention of nausea and vomiting associated with:1) initial and repeat courses of emetogenic cancer therapy, including high-dose cisplatin;2) radiation, including total body irradiation and fractionated abdominal radiation. Common adverse reactions include asthenia, headache, somnolence, and fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Granisetron hydrochloride tablets are indicated for the prevention of:
- Nausea and vomiting associated with initial and repeat courses of emetogenic cancer therapy, including high-dose cisplatin.
- Nausea and vomiting associated with radiation, including total body irradiation and fractionated abdominal radiation.
Dosage
Emetogenic Chemotherapy
The recommended adult dosage of oral granisetron hydrochloride tablets is 2 mg once daily or 1 mg twice daily. In the 2 mg once-daily regimen, two 1 mg tablets are given up to 1 hour before chemotherapy. In the 1 mg twice-daily regimen, the first 1 mg tablet is given up to 1 hour before chemotherapy, and the second tablet 12 hours after the first. Either regimen is administered only on the day(s) chemotherapy is given. Continued treatment, while not on chemotherapy, has not been found to be useful.
Radiation
The recommended adult dosage of oral granisetron hydrochloride tablets is 2 mg once daily. Two 1 mg tablets are taken within 1 hour of radiation.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Granisetron (tablet) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Granisetron (tablet) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indications
Granisetron hydrochloride tablets are indicated for the prevention of:
- Nausea and vomiting associated with initial and repeat courses of emetogenic cancer therapy, including high-dose cisplatin.
- Nausea and vomiting associated with radiation, including total body irradiation and fractionated abdominal radiation.
Dosage
Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Granisetron (tablet) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Granisetron (tablet) in pediatric patients.
Contraindications
Granisetron hydrochloride tablets are contraindicated in patients with known hypersensitivity to the drug or any of its components.
Warnings
Serotonin Syndrome
The development of serotonin syndrome has been reported with 5-HT3 receptor antagonists. Most reports have been associated with concomitant use of serotonergic drugs (e.g., selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), monoamine oxidase inhibitors, mirtazapine, fentanyl, lithium, tramadol, and intravenous methylene blue). Some of the reported cases were fatal. Serotonin syndrome occurring with overdose of another 5-HT3 receptor antagonist alone has also been reported. The majority of reports of serotonin syndrome related to 5-HT3 receptor antagonist use occurred in a post-anesthesia care unit or an infusion center.
Symptoms associated with serotonin syndrome may include the following combination of signs and symptoms: mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, with or without gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Patients should be monitored for the emergence of serotonin syndrome, especially with concomitant use of granisetron and other serotonergic drugs. If symptoms of serotonin syndrome occur, discontinue granisetron and initiate supportive treatment. Patients should be informed of the increased risk of serotonin syndrome, especially if granisetron is used concomitantly with other serotonergic drugs
Adverse Reactions
Clinical Trials Experience
Chemotherapy-Induced Nausea and Vomiting
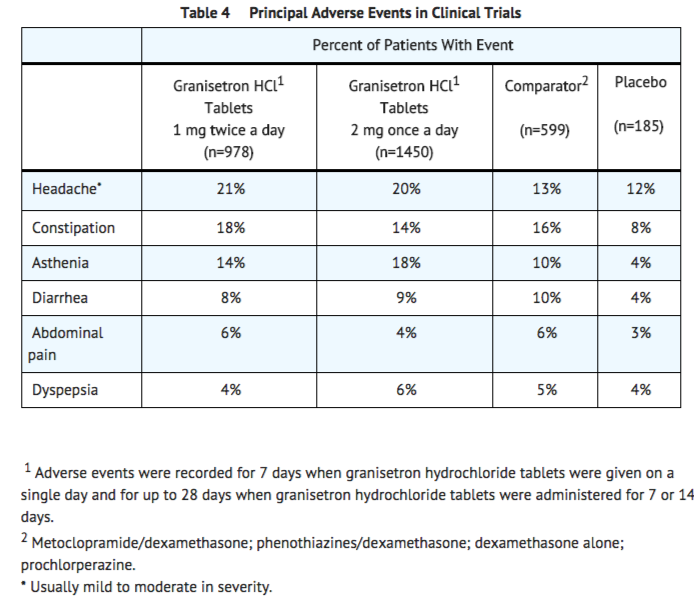
In patients receiving granisetron hydrochloride tablets 1 mg twice a day for 1, 7 or 14 days, or 2 mg once a day for 1 day, adverse experiences reported in more than 5% of the patients with comparator and placebo incidences are listed in Table 4.
Other adverse events reported in clinical trials were:
- Gastrointestinal: In single-day dosing studies in which adverse events were collected for 7 days, nausea (20%) and vomiting (12%) were recorded as adverse events after the 24-hour efficacy assessment period.
- Hepatic: In comparative trials, elevation of AST and ALT (>2 times the upper limit of normal) following the administration of granisetron hydrochloride tablets occurred in 5% and 6% of patients, respectively. These frequencies were not significantly different from those seen with comparators (AST: 2%; ALT: 9%).
- Cardiovascular: Hypertension (1%); hypotension, angina pectoris, atrial fibrillation, and syncope have been observed rarely.
- Central Nervous System: Dizziness (5%), insomnia (5%), anxiety (2%), somnolence (1%). One case compatible with, but not diagnostic of, extrapyramidal symptoms has been reported in a patient treated with granisetron hydrochloride tablets.
- Hypersensitivity: Rare cases of hypersensitivity reactions, sometimes severe (eg, anaphylaxis, shortness of breath, hypotension, urticaria) have been reported.
- Other: Fever (5%). Events often associated with chemotherapy also have been reported: leukopenia (9%), decreased appetite (6%), anemia (4%), alopecia (3%), thrombocytopenia (2%).
Over 5000 patients have received injectable granisetron hydrochloride in clinical trials.
Table 5 gives the comparative frequencies of the five commonly reported adverse events (≥ 3%) in patients receiving granisetron hydrochloride injection, 40 mcg/kg, in single-day chemotherapy trials. These patients received chemotherapy, primarily cisplatin, and intravenous fluids during the 24-hour period following granisetron hydrochloride injection administration.

In the absence of a placebo group, there is uncertainty as to how many of these events should be attributed to granisetron hydrochloride, except for headache, which was clearly more frequent than in comparison groups.
Radiation-Induced Nausea and Vomiting
In controlled clinical trials, the adverse events reported by patients receiving granisetron hydrochloride tablets and concurrent radiation were similar to those reported by patients receiving granisetron hydrochloride tablets prior to chemotherapy. The most frequently reported adverse events were diarrhea, asthenia, and constipation. Headache, however, was less prevalent in this patient population.
Postmarketing Experience
QT prolongation has been reported with granisetron hydrochloride.
Drug Interactions
Granisetron does not induce or inhibit the cytochrome P-450 drug-metabolizing enzyme system in vitro. There have been no definitive drug-drug interaction studies to examine pharmacokinetic or pharmacodynamic interaction with other drugs; however, in humans, granisetron hydrochloride injection has been safely administered with drugs representing benzodiazepines, neuroleptics, and anti-ulcer medications commonly prescribed with antiemetic treatments. Granisetron hydrochloride injection also does not appear to interact with emetogenic cancer chemotherapies. Because granisetron is metabolized by hepatic cytochrome P-450 drug-metabolizing enzymes, inducers or inhibitors of these enzymes may change the clearance and, hence, the half-life of granisetron. No specific interaction studies have been conducted in anesthetized patients. In addition, the activity of the cytochrome P-450 subfamily 3A4 (involved in the metabolism of some of the main narcotic analgesic agents) is not modified by granisetron hydrochloride in vitro.
In in vitro human microsomal studies, ketoconazole inhibited ring oxidation of granisetron hydrochloride. However, the clinical significance of in vivo pharmacokinetic interactions with ketoconazole is not known. In a human pharmacokinetic study, hepatic enzyme induction with phenobarbital resulted in a 25% increase in total plasma clearance of intravenous granisetron hydrochloride. The clinical significance of this change is not known.
QT prolongation has been reported with granisetron hydrochloride. Use of granisetron hydrochloride in patients concurrently treated with drugs known to prolong the QT interval and/or are arrhythmogenic, this may result in clinical consequences.
Serotonin syndrome (including altered mental status, autonomic instability, and neuromuscular symptoms) has been described following the concomitant use of 5-HT3 receptor antagonists and other serotonergic drugs, including selective serotonin reuptake inhibitors (SSRIs) and serotonin and noradrenaline reuptake inhibitors (SNRIs).
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B
Teratogenic Effect:
Reproduction studies have been performed in pregnant rats at oral doses up to 125 mg/kg/day (750 mg/m2/day, 507 times the recommended human dose based on body surface area) and pregnant rabbits at oral doses up to 32 mg/kg/day (378 mg/m2/day, 255 times the recommended human dose based on body surface area) and have revealed no evidence of impaired fertility or harm to the fetus due to granisetron. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS): B1
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Granisetron (tablet) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Granisetron (tablet) during labor and delivery.
Nursing Mothers
It is not known whether granisetron is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when granisetron hydrochloride is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- The ranges of the pharmacokinetic parameters in elderly volunteers (mean age 71 years), given a single 40 mcg/kg intravenous dose of granisetron hydrochloride injection, were generally similar to those in younger healthy volunteers; mean values were lower for clearance and longer for half-life in the elderly.
- During clinical trials, 325 patients 65 years of age or older received granisetron hydrochloride tablets; 298 were 65 to 74 years of age, and 27 were 75 years of age or older. Efficacy and safety were maintained with increasing age.
- For the prevention of nausea and vomiting of both metogenic chemotherapy and radiation, no dosage adjustment is recommended.
Gender
The effects of gender on the pharmacokinetics of granisteron hydrochloride tablets have not been studied. However, after intravenous infusion of granisetron hydrochloride, no difference in mean AUC was found between males and females, although males had a higher Cmax generally.
Race
There is no FDA guidance on the use of Granisetron (tablet) with respect to specific racial populations.
Renal Impairment
*Total clearance of granisetron was not affected in patients with severe renal failure who received a single 40 mcg/kg intravenous dose of granisetron hydrochloride injection.
- For the prevention of nausea and vomiting of both metogenic chemotherapy, no dosage adjustment is recommended.
Hepatic Impairment
- A pharmacokinetic study with intravenous granisetron hydrochloride in patients with hepatic impairment due to neoplastic liver involvement showed that total clearance was approximately halved compared to patients without hepatic impairment. Given the wide variability in pharmacokinetic parameters noted in patients, dosage adjustment in patients with hepatic functional impairment is not necessary.
- For the prevention of nausea and vomiting of both metogenic chemotherapy, no dosage adjustment is recommended.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Granisetron (tablet) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Granisetron (tablet) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Tablets for oral administration
Monitoring
There is limited information regarding Monitoring of Granisetron (tablet) in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Granisetron (tablet) and IV administrations.
Overdosage
There is no specific treatment for granisetron hydrochloride overdosage. In case of overdosage, symptomatic treatment should be given. Overdosage of up to 38.5 mg of granisetron hydrochloride injection has been reported without symptoms or only the occurrence of a slight headache.
Pharmacology
Mechanism of Action
There is limited information regarding Granisetron (tablet) Mechanism of Action in the drug label.
Structure
Granisetron hydrochloride tablets contain granisetron hydrochloride, an antinauseant and antiemetic agent. Chemically it is endo-N-(9-methyl-9-azabicyclo [3.3.1] non-3-yl)-1-methyl-1H-indazole-3-carboxamide hydrochloride with a molecular weight of 348.9 (312.4 free base). Its empirical formula is C18H24N4O•HCl while its chemical structure is:

Pharmacodynamics
Granisetron is a selective 5-hydroxytryptamine3 (5-HT3) receptor antagonist with little or no affinity for other serotonin receptors, including 5-HT1; 5-HT1A; 5-HT1B/C; 5-HT2; for alpha1-, alpha2-, or beta-adrenoreceptors; for dopamine-D2; or for histamine-H1; benzodiazepine; picrotoxin or opioid receptors.
Serotonin receptors of the 5-HT3 type are located peripherally on vagal nerve terminals and centrally in the chemoreceptor trigger zone of the area postrema. During chemotherapy that induces vomiting, mucosal enterochromaffin cells release serotonin, which stimulates 5-HT3 receptors. This evokes vagal afferent discharge, inducing vomiting. Animal studies demonstrate that, in binding to 5-HT3 receptors, granisetron blocks serotonin stimulation and subsequent vomiting after emetogenic stimuli such as cisplatin. In the ferret animal model, a single granisetron injection prevented vomiting due to high-dose cisplatin or arrested vomiting within 5 to 30 seconds.
In most human studies, granisetron has had little effect on blood pressure, heart rate or ECG. No evidence of an effect on plasma prolactin or aldosterone concentrations has been found in other studies.
Following single and multiple oral doses, granisetron hydrochloride tablets slowed colonic transit in normal volunteers. However, granisetron hydrochloride had no effect on oro-cecal transit time in normal volunteers when given as a single intravenous (IV) infusion of 50 mcg/kg or 200 mcg/kg.
Pharmacokinetics
In healthy volunteers and adult cancer patients undergoing chemotherapy, administration of granisetron hydrochloride tablets produced mean pharmacokinetic data shown in Table 1.

- Absorption: When granisetron hydrochloride tablets were administered with food, AUC was decreased by 5% and Cmax increased by 30% in non-fasted healthy volunteers who received a single dose of 10 mg.
- Distribution: Plasma protein binding is approximately 65% and granisetron distributes freely between plasma and red blood cells.
- Metabolism: Granisetron metabolism involves N-demethylation and aromatic ring oxidation followed by conjugation. In vitro liver microsomal studies show that granisetron's major route of metabolism is inhibited by ketoconazole, suggestive of metabolism mediated by the cytochrome P-450 3A subfamily. Animal studies suggest that some of the metabolites may also have 5-HT3 receptor antagonist activity.
- Elimination: Clearance is predominantly by hepatic metabolism. In normal volunteers, approximately 11% of the orally administered dose is eliminated unchanged in the urine in 48 hours. The remainder of the dose is excreted as metabolites, 48% in the urine and 38% in the feces.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Granisetron (tablet) in the drug label.
Clinical Studies
Chemotherapy-Induced Nausea and Vomiting
Granisetron hydrochloride tablets prevent nausea and vomiting associated with initial and repeat courses of emetogenic cancer therapy, as shown by 24-hour efficacy data from studies using both moderately-and highly-emetogenic chemotherapy.
Moderately Emetogenic Chemotherapy: The first trial compared granisteron hydrochloride tablet doses of 0.25 mg to 2 mg twice a day, in 930 cancer patients receiving, principally, cyclophosphamide, carboplatin, and cisplatin (20 mg/m2 to 50 mg/m2). Efficacy was based on complete response (ie, no vomiting, no moderate or severe nausea, no rescue medication), no vomiting, and no nausea. Table 2 summarizes the results of this study.

Results from a second double-blind, randomized trial evaluating granisetron hydrochloride tablets 2 mg once a day and granisetron hydrochloride tablets 1 mg twice a day were compared to prochlorperazine 10 mg twice a day derived from a historical control. At 24 hours, there was no statistically significant difference in efficacy between the two granisetron hydrochloride tablet regimens. Both regimens were statistically superior to the prochlorperazine control regimen (see Table 3).

Results from a granisetron hydrochloride tablets 2 mg once a day alone treatment arm in a third double-blind, randomized trial, were compared to prochlorperazine (PCPZ), 10 mg twice a day, derived from a historical control. The 24-hour results for granisetron hydrochloride tablets 2 mg once a day were statistically superior to PCPZ for all efficacy parameters: complete response (58%), no vomiting (79%), no nausea (51%), total control (49%). The PCPZ rates are shown in Table 3.
Cisplatin-Based Chemotherapy: The first double-blind trial compared granisetron hydrochloride tablets 1 mg twice a day, relative to placebo (historical control), in 119 cancer patients receiving high-dose cisplatin (mean dose 80 mg/m2). At 24 hours, granisetron hydrochloride tablets 1 mg twice a day was significantly (P <0.001) superior to placebo (historical control) in all efficacy parameters: complete response (52%), no vomiting (56%) and no nausea (45%). The placebo rates were 7%, 14%, and 7%, respectively, for the three efficacy parameters.
Results from a granisetron hydrochloride tablets 2 mg once a day alone treatment arm in a second double-blind, randomized trial, were compared to both granisetron hydrochloride tablets 1 mg twice a day and placebo historical controls. The 24-hour results for granisetron hydrochloride tablets 2 mg once a day were: complete response (44%), no vomiting (58%), no nausea (46%), total control (40%). The efficacy of granisetron hydrochloride tablets 2 mg once a day was comparable to granisetron hydrochloride tablets 1 mg twice a day and statistically superior to placebo. The placebo rates were 7%, 14%, 7%, and 7%, respectively, for the four parameters.
No controlled study comparing granisetron injection with the oral formulation to prevent chemotherapy-induced nausea and vomiting has been performed.
Radiation-Induced Nausea and Vomiting
Total Body Irradiation: In a double-blind randomized study, 18 patients receiving granisetron hydrochloride tablets, 2 mg daily, experienced significantly greater antiemetic protection compared to patients in a historical negative control group who received conventional (non-5-HT3 antagonist) antiemetics. Total body irradiation consisted of 11 fractions of 120 cGy administered over 4 days, with three fractions on each of the first 3 days, and two fractions on the fourth day. Granisetron hydrochloride tablets were given one hour before the first radiation fraction of each day.
Twenty-two percent (22%) of patients treated with granisetron hydrochloride tablets did not experience vomiting or receive rescue antiemetics over the entire 4-day dosing period, compared to 0% of patients in the historical negative control group (P <0.01).
In addition, patients who received granisetron hydrochloride tablets also experienced significantly fewer emetic episodes during the first day of radiation and over the 4-day treatment period, compared to patients in the historical negative control group. The median time to the first emetic episode was 36 hours for patients who received granisetron hydrochloride tablets.
Fractionated Abdominal Radiation: The efficacy of granisetron hydrochloride tablets, 2 mg daily, was evaluated in a double-blind, placebo-controlled randomized trial of 260 patients. Granisetron hydrochloride tablets were given 1 hour before radiation, composed of up to 20 daily fractions of 180 to 300 cGy each. The exceptions were patients with seminoma or those receiving whole abdomen irradiation who initially received 150 cGy per fraction. Radiation was administered to the upper abdomen with a field size of at least 100 cm2.
The proportion of patients without emesis and those without nausea for granisetron hydrochloride tablets, compared to placebo, was statistically significant (P <0.0001) at 24 hours after radiation, irrespective of the radiation dose. Granisetron hydrochloride was superior to placebo in patients receiving up to 10 daily fractions of radiation, but was not superior to placebo in patients receiving 20 fractions.
Patients treated with granisetron hydrochloride tablets (n=134) had a significantly longer time to the first episode of vomiting (35 days vs. 9 days, P <0.001) relative to those patients who received placebo (n=126), and a significantly longer time to the first episode of nausea (11 days vs. 1 day, P <0.001). Granisetron hydrochloride provided significantly greater protection from nausea and vomiting than placebo.
How Supplied
Granisetron hydrochloride tablets 1 mg are white round film coated tablets debossed cor over 198 on one side and plain on the other side.
They are supplied as follows: Blister cards of 2 unit of use tablets: NDC 64720-198-98 Blister cards of 20 unit of use tablets: NDC 64720-198-97 (intended for institutional use only) Bottles of 20: NDC 64720-198-02 (intended for institutional use only) Bottles of 100: NDC 64720-198-10 (intended for institutional use only)
Storage
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Keep container closed tightly. Protect from light.
Keep this and all drugs out of the reach of children.
Images
Drug Images
{{#ask: Page Name::Granisetron (tablet) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Granisetron (tablet) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Advise patients of the possibility of serotonin syndrome with concomitant use of granisetron and another serotonergic agent such as medications to treat depression and migraines. Advise patients to seek immediate medical attention if the following symptoms occur: changes in mental status, autonomic instability, neuromuscular symptoms with or without gastrointestinal symptoms.
Precautions with Alcohol
- Alcohol-Granisetron (tablet) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- GRANISETRON HYDROCHLORIDE®[1]
Look-Alike Drug Names
There is limited information regarding Granisetron (tablet) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.