Glioblastoma multiforme pathophysiology
|
Glioblastoma multiforme Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Glioblastoma multiforme pathophysiology On the Web |
|
American Roentgen Ray Society Images of Glioblastoma multiforme pathophysiology |
|
Risk calculators and risk factors for Glioblastoma multiforme pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]Associate Editor(s)-in-Chief: Sujit Routray, M.D. [2]
Overview
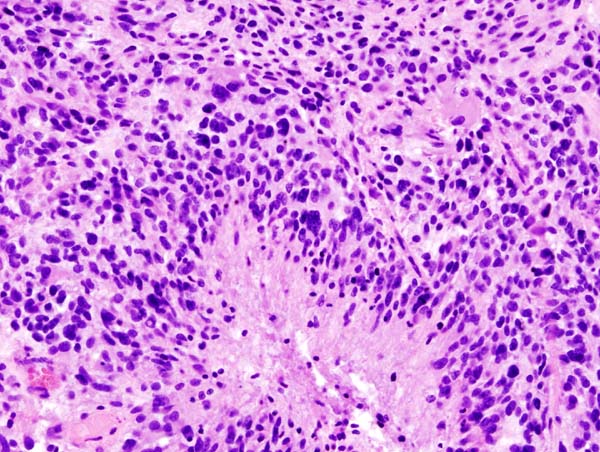
Glioblastoma is characterized by the presence of small areas of necrotizing tissue that are surrounded by anaplastic cells. Genes involved in the pathogenesis of glioblastoma include Mdm2, PTEN, IDH1, p53, and chromosomes 10p, 10q, 17p, and 19q. On gross pathology, the characteristic findings of glioblastoma include firm and white, to soft and yellow, poorly-marginated, diffusely infiltrating, ill-defined borders, firm or gelatinous mass with central necrotic core. On microscopic histopathological analysis, the characteristic findings of glioblastoma include pleomorphic astrocytes with marked atypia, mitosis, necrosis, and microvascular proliferation.[1]
Pathophysiology
Pathogenesis
Glioblastoma is characterized by the presence of small areas of necrotizing tissue that are surrounded by anaplastic cells. Presence of hyperplastic blood vessels differentiates glioblastoma from grade 3 astrocytomas.
Molecular alterations
- There are four subtypes of glioblastoma.[2]
- Tumors in the "classical" subtype carry extra copies of the epidermal growth factor receptor (EGFR) gene, and most have higher than normal expression of epidermal growth factor receptor (EGFR), whereas the gene TP53, which is often mutated in glioblastoma, is rarely mutated in this subtype.
- The "proneural" subtype often has high rates of alterations in TP53, and in PDGFRA, the gene encoding a-type platelet-derived growth factor receptor, and in IDH1, the gene encoding isocitrate dehydrogenase-1.
- The "mesenchymal" subtype is characterized by high rates of mutations or other alterations in NF1, the gene encoding Neurofibromin 1 and fewer alterations in the EGFR gene and less expression of EGFR than other types. Many other genetic alterations have been described in glioblastoma, and the majority of them are clustered in three pathways, the p53, Rb, and PI3K/AKT. Glioblastomas have alterations in 64-87 %, 68-78 % and 88% of these pathways respectively. Another important alteration is methylation of MGMT, a suicide DNA repair enzyme. Methylation is described to impair DNA transcription and therefore, expression of the MGMT enzyme. Since MGMT enzyme can only repair one DNA alkylation due its suicide repair mechanism, reverse capacity is low and methylation of the MGMT gene promoter greatly affects DNA repair capacity. Indeed, MGMT methylation is associated with an improved response to treatment with DNA-damaging chemotherapeutics, such as temozolomide.
- The "neural" subtype has several mutations in many of the same genes as the other groups. The prognosis is worse with aggressive treatment with respect to classical and mesenchymal subtypes.
- Glioblastomas usually form in the cerebral white matter, grow quickly, and can become very large before producing symptoms. Less than 10% form more slowly following degeneration of low-grade astrocytoma or anaplastic astrocytoma. These are called secondary glioblastomas and are more common in younger patients (mean age 45 versus 62 years).
- The tumor may extend into the meninges or ventricular wall, leading to high protein content in the cerebrospinal fluid (CSF) (> 100 mg/dL), as well as an occasional pleocytosis of 10 - 100 cells, mostly lymphocytes. Malignant cells carried in the CSF may rarely spread to the spinal cord or cause meningeal gliomatosis. However, metastasis of glioblastoma beyond the central nervous system is extremely unusual. About 50% of glioblastomas occupy more than one lobe of a hemisphere or are bilateral. Tumors of this type usually arise from the cerebrum and may rarely exhibit the classic infiltration across the corpus callosum, producing a butterfly (bilateral) glioma.
- The tumor may take on a variety of appearances, depending on the amount of hemorrhage, necrosis, or its age. .
Glioblastoma stem-like cells
- Cancer cells with stem cell-like properties have been found in glioblastomas, which may be a cause of their resistance to conventional treatment and high recurrence rate).
- A biomarker that exhibits cancer stem cell properties, Hes3, has been shown to regulate cells of glioblastoma when placed in culture.
Metabolism
- The IDH1 gene encodes for the enzyme isocitrate dehydrogenase 1 and is frequently mutated in glioblastoma (primary: 5%, secondary: 80%). By producing very high concentrations of the oncometabolite D-2-hydroxyglutarate and dysregulating the function of the wild-type IDH1-enzyme, it induces profound changes to the metabolism of IDH1-mutated glioblastoma compared with IDH1 wild-type glioblastoma or healthy astrocytes. *Among others, it increases the dependence of glioblastoma on glutamine or glutamate as an energy source.
- It has been hypothesized that IDH1-mutated glioblastoma use glutamate as a chemotactic signal. Since healthy astrocytes excrete glutamate, IDH1-mutated glioblastoma cells do not favor dense tumor structures but instead migrate, invade and disperse into healthy parts of the brain where glutamate concentrations are higher. This may explain the invasive behaviour of these IDH1-mutated glioblastoma.
Ion channels
- Glioblastoma exhibits numerous alterations in genes that encode for ion channels, including upregulation of gBK potassium channels and ClC-3 chloride channels.
- It has been hypothesized that by upregulating these ion channels, glioblastoma tumor cells can facilitate increased ion movement over the cell membrane, thereby increasing H2O movement through osmosis, which aids glioblastoma cells in changing cellular volume very rapidly. This is helpful in their extremely aggressive invasive behavior, because quick adaptations in cellular volume can facilitate movement through the extracellular matrix of the brain.
Genetics
- Development of glioblastoma is the result from multiple genetic mutations.
- Genes involved in the pathogenesis of glioblastoma include the following:[1]
| Types of glioblastoma | Genes |
|---|---|
|
|
|
|
Associated Conditions
Glioblastoma may be associated with:[1]
- Neurofibromatosis type 1
- Li-Fraumeni syndrome
- Turcot syndrome
- Ollier disease
- Maffucci syndrome
- Tuberous sclerosis
- Von Hippel-Lindau disease
Gross Pathology
On gross pathology, the characteristic findings of glioblastomas include:[1][3]
- Supratentorial white matter is the most common location
- Poorly-marginated, diffusely infiltrating mass with central necrotic core
- Ill-defined borders
- Firm or gelatinous in consistency
- Variable appearance (firm and white, to soft and yellow, to cystic with hemorrhage)
- Midline shift due to tumor mass
- Bihemispheric "butterfly glioma" in the corpus callosum
Microscopic Pathology
On microscopic histopathological analysis, the characteristic findings of glioblastomas include:[1][3]
- Pleomorphic astrocytes with marked atypia and mitosis
- Necrosis and microvascular proliferation
- (+/-) Pseudopalisading necrosis
- Tumor cells lined-up like a picket fence around necrotic areas
According to WHO classification of brain tumors, glioblastoma is termed as grade IV tumor.[1]
Markers
Glioblastoma is demonstrated by positivity to tumor marker such as GFAP.[1]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Pathology of glioblastoma multiforme. Dr Dylan Kurda and Dr Frank Gaillard et al. Radiopaedia 2015. http://radiopaedia.org/articles/glioblastoma
- ↑ Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD; et al. (2010). "Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1". Cancer Cell. 17 (1): 98–110. doi:10.1016/j.ccr.2009.12.020. PMC 2818769. PMID 20129251.
- ↑ 3.0 3.1 Pathology of glioblastoma multiforme. Libre Pathology. http://librepathology.org/wiki/index.php/Glioblastoma