Gemifloxacin mesylate: Difference between revisions
Kiran Singh (talk | contribs) No edit summary |
Kiran Singh (talk | contribs) No edit summary |
||
| Line 27: | Line 27: | ||
FACTIVE can be taken with or without food and should be swallowed whole with a liberal amount of liquid. The recommended dose of FACTIVE is 320 mg daily, according to the following table (Table 4). | FACTIVE can be taken with or without food and should be swallowed whole with a liberal amount of liquid. The recommended dose of FACTIVE is 320 mg daily, according to the following table (Table 4). | ||
''' | '''Recommended Dosage Regimen of FACTIVE''' | ||
* The clinical decision regarding the use of a 5 or 7 day regimen should be guided by results of the initial sputum culture. | * The clinical decision regarding the use of a 5 or 7 day regimen should be guided by results of the initial sputum culture. | ||
[[File: | [[File:Gemifloxacin table4.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
The recommended dose and duration of FACTIVE should not be exceeded. | The recommended dose and duration of FACTIVE should not be exceeded. | ||
| Line 37: | Line 37: | ||
'''Use in Renally Impaired Patients''': Dose adjustment in patients with creatinine clearance >40 mL/min is not required. Modification of the dosage is recommended for patients with creatinine clearance ≤40 mL/min. Table 5 provides dosage guidelines for use in patients with renal impairment. | '''Use in Renally Impaired Patients''': Dose adjustment in patients with creatinine clearance >40 mL/min is not required. Modification of the dosage is recommended for patients with creatinine clearance ≤40 mL/min. Table 5 provides dosage guidelines for use in patients with renal impairment. | ||
[[File: | [[File:Gemifloxacin table5.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
* Patients requiring routine hemodialysis or continuous ambulatory peritoneal dialysis (CAPD) should receive 160 mg every 24 hours. | * Patients requiring routine hemodialysis or continuous ambulatory peritoneal dialysis (CAPD) should receive 160 mg every 24 hours. | ||
| Line 43: | Line 43: | ||
* When only the serum creatinine concentration is known, the following formula may be used to estimate creatinine clearance. | * When only the serum creatinine concentration is known, the following formula may be used to estimate creatinine clearance. | ||
[[File:Gemifloxacin formula.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
'''Creatinine Clearance Formula''' | '''Creatinine Clearance Formula''' | ||
| Line 48: | Line 49: | ||
Women: 0.85 x the value calculated for men | Women: 0.85 x the value calculated for men | ||
Use in Hepatically Impaired Patients: No dosage adjustment is recommended in patients with mild (Child-Pugh Class A), moderate (Child-Pugh Class B) or severe (Child-Pugh Class C) hepatic impairment. | '''Use in Hepatically Impaired Patients''': No dosage adjustment is recommended in patients with mild (Child-Pugh Class A), moderate (Child-Pugh Class B) or severe (Child-Pugh Class C) hepatic impairment. | ||
Use in Elderly: No dosage adjustment is recommended. | '''Use in Elderly''': No dosage adjustment is recommended. | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
| | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed= | |fdaLIADPed=There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | ||
There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
< | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|clinicalTrials=There is limited information regarding <i>Clinical Trial Experience</i> of {{PAGENAME}} in the drug label. | |clinicalTrials=There is limited information regarding <i>Clinical Trial Experience</i> of {{PAGENAME}} in the drug label. | ||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | ||
|useInPregnancyFDA=* '''Pregnancy Category''' | |useInPregnancyFDA=* '''Pregnancy Category''' | ||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | ||
| Line 288: | Line 103: | ||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | ||
= | |overdose=There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | ||
<!--Drug box 2--> | <!--Drug box 2--> | ||
| Line 310: | Line 113: | ||
<!--Structure--> | <!--Structure--> | ||
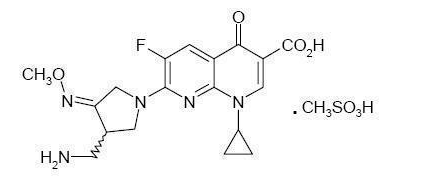
|structure=* | |structure=* FACTIVE (gemifloxacin mesylate) is a synthetic broad-spectrum antibacterial agent for oral administration. Gemifloxacin, a compound related to the fluoroquinolone class of antibiotics, is available as the mesylate salt in the sesquihydrate form. Chemically, gemifloxacin is (R,S)-7-[(4Z)-3-(aminomethyl)-4-(methoxyimino)-1-pyrrolidinyl]-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid. | ||
* The mesylate salt is a white to light brown solid with a molecular weight of 485.49. Gemifloxacin is considered freely soluble at neutral pH (350 μg/mL at 37ºC, pH 7.0). Its empirical formula is C18H20FN5O4•CH4O3S and its chemical structure is: | |||
[[File:Gemifloxacin structure.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
* Each white to off-white, oval, film-coated FACTIVE tablet has breaklines and GE 320 debossed on both faces and contains gemifloxacin mesylate equivalent to 320 mg gemifloxacin. The inactive ingredients are crospovidone, hydroxypropyl methylcellulose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, and titanium dioxide. | |||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | ||
|nonClinToxic=There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | |nonClinToxic=There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | ||
Revision as of 18:09, 13 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING:
See full prescribing information for complete Boxed Warning.
* Fluoroquinolones, including FACTIVE®, are associated with an increased risk of tendinitis and tendon rupture in all ages. This risk is further increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart and lung transplants.
|
Overview
Gemifloxacin mesylate is an antibiotic that is FDA approved for the treatment of {{{indication}}}. There is a Black Box Warning for this drug as shown here. Common adverse reactions include rash, abdominal pain, diarrhea, nausea, vomiting,dizziness, headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
FACTIVE is indicated for the treatment of infections caused by susceptible strains of the designated microorganisms in the conditions listed below.
Acute bacterial exacerbation of chronic bronchitis caused by Streptococcus pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae, or Moraxella catarrhalis.
Community-acquired pneumonia (of mild to moderate severity) caused by Streptococcus pneumoniae (including multi-drug resistant strains [MDRSP])*, Haemophilus influenzae, Moraxella catarrhalis, Mycoplasma pneumoniae, Chlamydia pneumoniae, or Klebsiella pneumoniae.
MDRSP: multi-drug resistant Streptococcus pneumoniae, includes isolates previously known as PRSP (penicillin-resistant Streptococcus pneumoniae), and are strains resistant to two or more of the following antibiotics: penicillin (MIC ≥2 μg/mL), 2nd generation cephalosporins (e.g., cefuroxime), macrolides, tetracyclines and trimethoprim/sulfamethoxazole.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of FACTIVE and other antibacterial drugs, FACTIVE should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Dosage
FACTIVE can be taken with or without food and should be swallowed whole with a liberal amount of liquid. The recommended dose of FACTIVE is 320 mg daily, according to the following table (Table 4).
Recommended Dosage Regimen of FACTIVE
- The clinical decision regarding the use of a 5 or 7 day regimen should be guided by results of the initial sputum culture.

The recommended dose and duration of FACTIVE should not be exceeded.
Use in Renally Impaired Patients: Dose adjustment in patients with creatinine clearance >40 mL/min is not required. Modification of the dosage is recommended for patients with creatinine clearance ≤40 mL/min. Table 5 provides dosage guidelines for use in patients with renal impairment.

- Patients requiring routine hemodialysis or continuous ambulatory peritoneal dialysis (CAPD) should receive 160 mg every 24 hours.
- When only the serum creatinine concentration is known, the following formula may be used to estimate creatinine clearance.

Creatinine Clearance Formula
Women: 0.85 x the value calculated for men
Use in Hepatically Impaired Patients: No dosage adjustment is recommended in patients with mild (Child-Pugh Class A), moderate (Child-Pugh Class B) or severe (Child-Pugh Class C) hepatic impairment.
Use in Elderly: No dosage adjustment is recommended.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Gemifloxacin mesylate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Gemifloxacin mesylate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Gemifloxacin mesylate in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Gemifloxacin mesylate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Gemifloxacin mesylate in pediatric patients.
Contraindications
There is limited information regarding Gemifloxacin mesylate Contraindications in the drug label.
Warnings
|
WARNING:
See full prescribing information for complete Boxed Warning.
* Fluoroquinolones, including FACTIVE®, are associated with an increased risk of tendinitis and tendon rupture in all ages. This risk is further increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart and lung transplants.
|
There is limited information regarding Gemifloxacin mesylate Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Gemifloxacin mesylate in the drug label.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Gemifloxacin mesylate in the drug label.
Drug Interactions
There is limited information regarding Gemifloxacin mesylate Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Gemifloxacin mesylate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Gemifloxacin mesylate during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Gemifloxacin mesylate with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Gemifloxacin mesylate with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Gemifloxacin mesylate with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Gemifloxacin mesylate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Gemifloxacin mesylate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Gemifloxacin mesylate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Gemifloxacin mesylate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Gemifloxacin mesylate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Gemifloxacin mesylate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Gemifloxacin mesylate in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Gemifloxacin mesylate in the drug label.
Overdosage
There is limited information regarding Chronic Overdose of Gemifloxacin mesylate in the drug label.
Pharmacology
There is limited information regarding Gemifloxacin mesylate Pharmacology in the drug label.
Mechanism of Action
Structure
- FACTIVE (gemifloxacin mesylate) is a synthetic broad-spectrum antibacterial agent for oral administration. Gemifloxacin, a compound related to the fluoroquinolone class of antibiotics, is available as the mesylate salt in the sesquihydrate form. Chemically, gemifloxacin is (R,S)-7-[(4Z)-3-(aminomethyl)-4-(methoxyimino)-1-pyrrolidinyl]-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid.
- The mesylate salt is a white to light brown solid with a molecular weight of 485.49. Gemifloxacin is considered freely soluble at neutral pH (350 μg/mL at 37ºC, pH 7.0). Its empirical formula is C18H20FN5O4•CH4O3S and its chemical structure is:
- Each white to off-white, oval, film-coated FACTIVE tablet has breaklines and GE 320 debossed on both faces and contains gemifloxacin mesylate equivalent to 320 mg gemifloxacin. The inactive ingredients are crospovidone, hydroxypropyl methylcellulose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, and titanium dioxide.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Gemifloxacin mesylate in the drug label.
Pharmacokinetics
There is limited information regarding Gemifloxacin mesylate Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Gemifloxacin mesylate in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Gemifloxacin mesylate in the drug label.
How Supplied
Storage
There is limited information regarding Gemifloxacin mesylate Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Gemifloxacin mesylate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Gemifloxacin mesylate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Gemifloxacin mesylate in the drug label.
Precautions with Alcohol
- Alcohol-Gemifloxacin mesylate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Gemifloxacin mesylate
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Gemifloxacin mesylate |Label Name=Gemifloxacin mesylate11.png
}}
{{#subobject:
|Label Page=Gemifloxacin mesylate |Label Name=Gemifloxacin mesylate11.png
}}