Fosamprenavir contraindications
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
Contraindications
LEXIVA is contraindicated:
- In patients with previously demonstrated clinically significant hypersensitivity (e.g., Stevens-Johnson syndrome) to any of the components of this product or to amprenavir.
- When coadministered with drugs that are highly dependent on cytochrome P450 3A4 (CYP3A4) for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events (Table 2).[1]
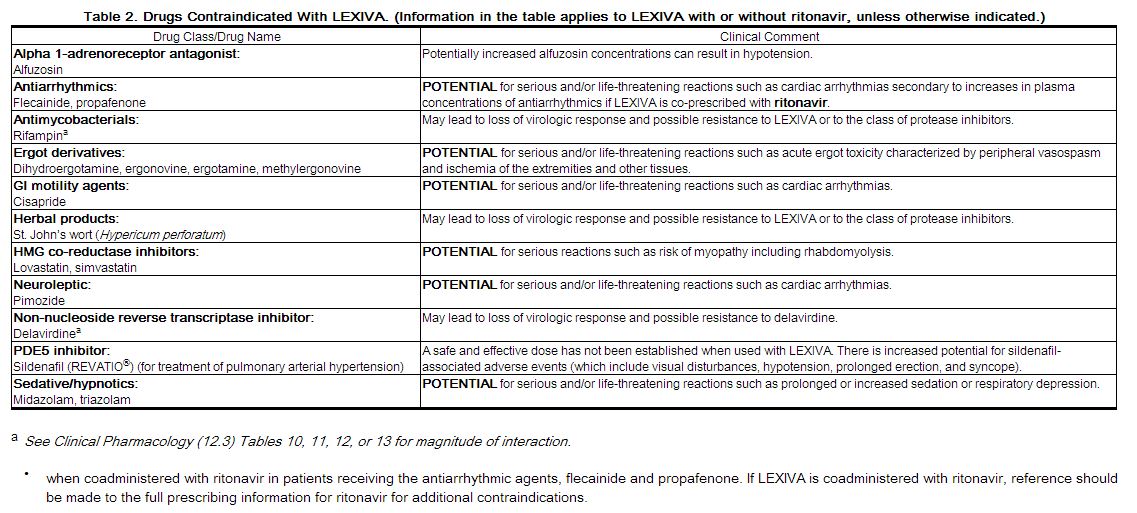 |
References
Adapted from the FDA Package Insert.