Flunarizine: Difference between revisions
m (Protected "Flunarizine": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
Rabin Bista (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
{{Drugbox| | {{Drugbox | ||
|IUPAC_name = 1-[bis(4-fluorophenyl)methyl]-4- | | Watchedfields = changed | ||
| image=Flunarizine | | verifiedrevid = 443822137 | ||
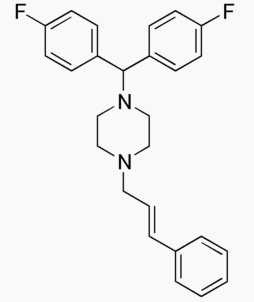
| IUPAC_name = 1-[''bis''(4-fluorophenyl)methyl]-4-[(2''E'')-3-phenylprop-2-en-1-yl]piperazine | |||
| | | image = Flunarizine Wiki Str.png | ||
| | |||
| | |||
| | <!--Clinical data--> | ||
| | | tradename = | ||
| Drugs.com = {{drugs.com|CONS|flunarizine}} | |||
| pregnancy_category = | |||
| bioavailability= | | legal_status = | ||
| metabolism = | | routes_of_administration = | ||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| metabolism = | |||
| excretion = | | excretion = | ||
| | |||
| | <!--Identifiers--> | ||
| | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 52468-60-7 | |||
| ATC_prefix = N07 | |||
| ATC_suffix = CA03 | |||
| PubChem = 941361 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB04841 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 819216 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = R7PLA2DM0J | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D07971 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 30008 | |||
<!--Chemical data--> | |||
| C=26 | H=26 | F=2 | N=2 | |||
| molecular_weight = 404.495 | |||
| smiles = Fc1ccc(cc1)C(c2ccc(F)cc2)N3CCN(CC3)C\C=C\c4ccccc4 | |||
| InChI = 1/C26H26F2N2/c27-24-12-8-22(9-13-24)26(23-10-14-25(28)15-11-23)30-19-17-29(18-20-30)16-4-7-21-5-2-1-3-6-21/h1-15,26H,16-20H2/b7-4+ | |||
| InChIKey = SMANXXCATUTDDT-QPJJXVBHBH | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C26H26F2N2/c27-24-12-8-22(9-13-24)26(23-10-14-25(28)15-11-23)30-19-17-29(18-20-30)16-4-7-21-5-2-1-3-6-21/h1-15,26H,16-20H2/b7-4+ | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = SMANXXCATUTDDT-QPJJXVBHSA-N | |||
| synonyms = <small>1-[''bis''(4-fluorophenyl)methyl]-4-cinnamyl-piperazine</small> | |||
}} | }} | ||
'''Flunarizine''' is a drug classified as a [[calcium channel blocker]]. Flunarizine is a selective calcium entry blocker with | '''Flunarizine''' is a drug classified as a [[calcium channel blocker]].<ref>[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1987188/ Calium channel blocker]</ref> Flunarizine is a non-selective calcium entry blocker with other actions including [[histamine]] H<sub>1</sub> receptor blocking activity. It is effective in the prophylaxis of [[migraine]],<ref>[http://www.ncbi.nlm.nih.gov/pubmed/6343298 Effective in migraine]</ref> occlusive [[peripheral vascular disease]], [[vertigo (medical)|vertigo]] of central and peripheral origin, and as an adjuvant in the therapy of [[epilepsy]]. It may help to reduce the severity and duration of attacks of paralysis associated with the more serious form of [[alternating hemiplegia]], as well as being effective in rapid onset dystonia-parkinsonism (RDP). Both these conditions share a mutation in the ATP13A gene; flunarizine is not available by prescription in the U.S. or Japan.<ref>[http://www.neurology.org/content/82/6/468.full ATP13A mutation,and response to flunarizine]</ref> Flunarizine has been shown to significantly reduce headache frequency and severity in both adults and children. Flunarizine was discovered at [[Janssen Pharmaceutica]] in 1968. | ||
==Side effects and contraindications== | |||
Flunarizine has some side effects including weight gain, extrapyramidal effects, drowsiness and [[Depression (mood)|depression]], it is [[contraindicated]] in hypotension, heart failure and arrhythmia. | |||
Flunarizine use is avoided in patients with depression, severe [[constipation]] or those with [[extrapyramidal system|extrapyramidal]] disorders. | |||
==References== | ==References== | ||
* | {{reflist}} | ||
==External References== | |||
* Therapeutic Choices, sixth edition, [[Canadian Pharmacists Association]], 2011. | |||
{{Antivertigo preparations}} | |||
{{Channel blockers}} | |||
{{Depressogenics}} | |||
[[Category:Calcium channel blockers]] | [[Category:Calcium channel blockers]] | ||
{{ | [[Category:Depressogenics]] | ||
{{ | [[Category:Piperazines]] | ||
[[Category:Organofluorides]] | |||
[[Category:Janssen Pharmaceutica]] | |||
[[Category:Belgian inventions]] | |||
{{cardiovascular-drug-stub}} | |||
{{nervous-system-drug-stub}} | |||
Latest revision as of 20:02, 6 April 2015
 | |
| Clinical data | |
|---|---|
| Synonyms | 1-[bis(4-fluorophenyl)methyl]-4-cinnamyl-piperazine |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C26H26F2N2 |
| Molar mass | 404.495 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Flunarizine is a drug classified as a calcium channel blocker.[1] Flunarizine is a non-selective calcium entry blocker with other actions including histamine H1 receptor blocking activity. It is effective in the prophylaxis of migraine,[2] occlusive peripheral vascular disease, vertigo of central and peripheral origin, and as an adjuvant in the therapy of epilepsy. It may help to reduce the severity and duration of attacks of paralysis associated with the more serious form of alternating hemiplegia, as well as being effective in rapid onset dystonia-parkinsonism (RDP). Both these conditions share a mutation in the ATP13A gene; flunarizine is not available by prescription in the U.S. or Japan.[3] Flunarizine has been shown to significantly reduce headache frequency and severity in both adults and children. Flunarizine was discovered at Janssen Pharmaceutica in 1968.
Side effects and contraindications
Flunarizine has some side effects including weight gain, extrapyramidal effects, drowsiness and depression, it is contraindicated in hypotension, heart failure and arrhythmia. Flunarizine use is avoided in patients with depression, severe constipation or those with extrapyramidal disorders.
References
External References
- Therapeutic Choices, sixth edition, Canadian Pharmacists Association, 2011.
Template:Antivertigo preparations Template:Channel blockers Template:Depressogenics
Template:Cardiovascular-drug-stub Template:Nervous-system-drug-stub
- Pages with script errors
- Template:drugs.com link with non-standard subpage
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Drugs with no legal status
- Drugboxes which contain changes to watched fields
- Calcium channel blockers
- Depressogenics
- Piperazines
- Organofluorides
- Janssen Pharmaceutica
- Belgian inventions