Fibroma pathophysiology: Difference between revisions
Simrat Sarai (talk | contribs) |
Simrat Sarai (talk | contribs) No edit summary |
||
| Line 323: | Line 323: | ||
{{WS}} | {{WS}} | ||
[[Category:Gynecology]] | [[Category:Gynecology]] | ||
[[Category:Dermatology]] | |||
[[Category:Oncology]] | |||
[[Category:Types of cancer]] | [[Category:Types of cancer]] | ||
Revision as of 18:23, 23 March 2016
|
Fibroma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Fibroma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Fibroma pathophysiology |
|
Risk calculators and risk factors for Fibroma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]Associate Editor(s)-in-Chief: Simrat Sarai, M.D. [2]
Overview
On gross pathology, polypoid lesion which is usually small, is characteristic findings of oral fibroma. On microscopic histopathological analysis, fibrous stroma, collagen bundles, prominent vessels, and overlying squamous mucosa with hyperkeratosis and focal ulceration are characteristic findings of oral fibroma. On gross pathology, well circumscribed, metaphyseal lesion, and fragments of white-grey rubbery tissue are characteristic findings of chondromyxoid fibroma. On microscopic histopathological analysis, spindle cells or stellate cells in a myxoid or chondroid stroma, lobules with hypocellular centers and hypercellular peripheries, giant cells in a hypercellular periphery, and scattered calcifications are characteristic findings of chondromyxoid fibroma. On gross pathology, fleshy, fibrous, yellow or tan-brown lesion with variable areas of haemorrhage are characteristic findings of non-ossifying fibroma. On microscopic histopathological analysis, spindle cells without cytologic atypia are arranged in a storiform pattern, scattered chronic inflammatory cells and benign giant cells, foam cells and hemosiderin deposition, and mitoses are characteristic findings of non-ossifying fibroma. On gross pathology, discrete mass that is well delineated from surrounding bone, tan-white, rubbery cut surface, firm to gritty and no encapsulation are characteristic findings of ossifying fibroma. On microscopic histopathological analysis, haphazardly distributed lamellated bony spicules on a background of fibrous stroma, a zonal architecture with a center of immature bone surrounded by more mature lamellar bone, and central spicules of woven bony trabeculae are lined by a layer of osteoblasts are characteristic findings of ossifying fibroma.[1][2]
Pathophysiology
Ovarian Fibroma
Associated Conditions
- Uterine fibromas are associated with ascites in approximately 40% of cases and with pleural effusions in a small percentage of cases.
- Meigs syndrome: consists of an ovarian fibroma with ascites and a pleural effusion
- Fibromas are seen in approximately 75% of patients with nevoid basal cell carcinoma syndrome (Gorlin syndrome).
Gross Pathology
On gross pathology, solid white mass which is usually well-circumscribed is characteristic findings of ovarian fibroma.
Microscopic Pathology
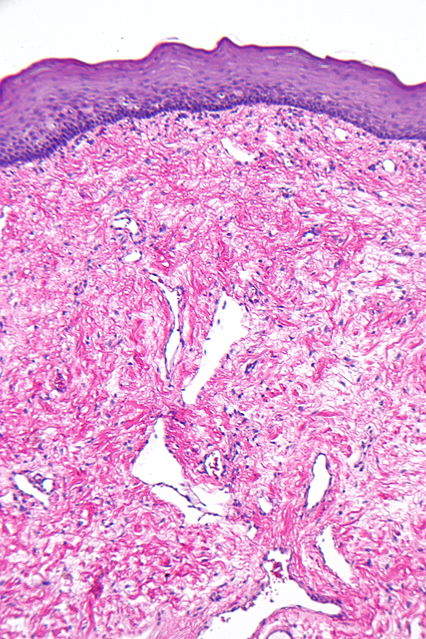
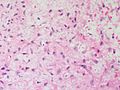
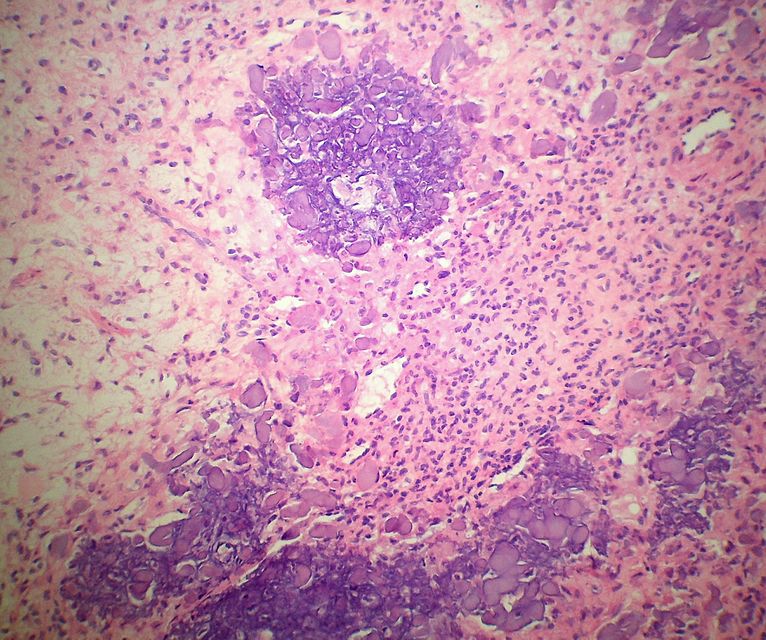
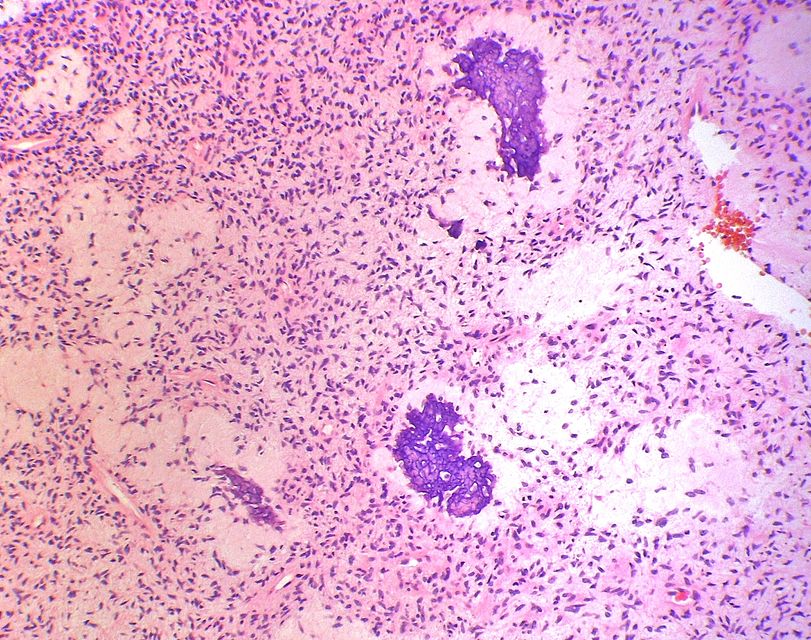
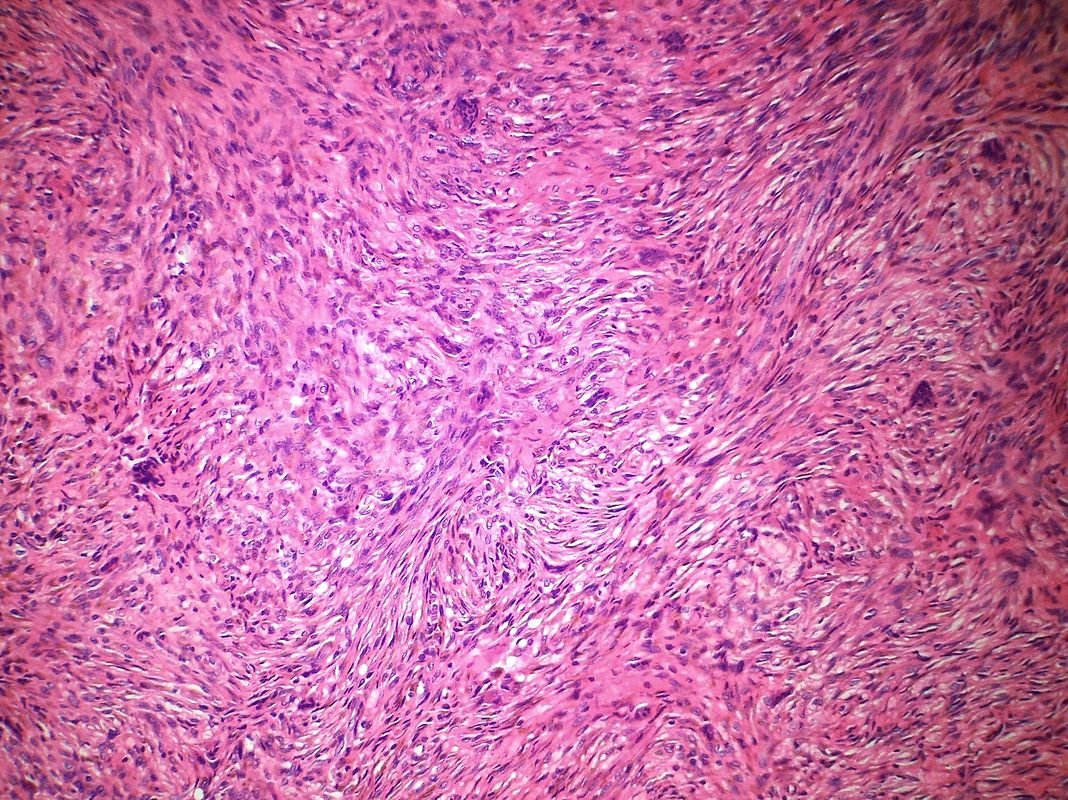
On microscopic histopathological analysis, fibroma is composed of spindle cells forming variable amounts of collagen. Sectioning of a fibroma typically reveals a chalky-white surface that has a whorled appearance, similar to that of a uterine fibroid. Areas of edema, occasionally with cyst formation, are also relatively common.
-
Histopathology specimen of an ovarian fibroma high magnification
-
Histopathology specimen of an ovarian fibroma intermediate magnification
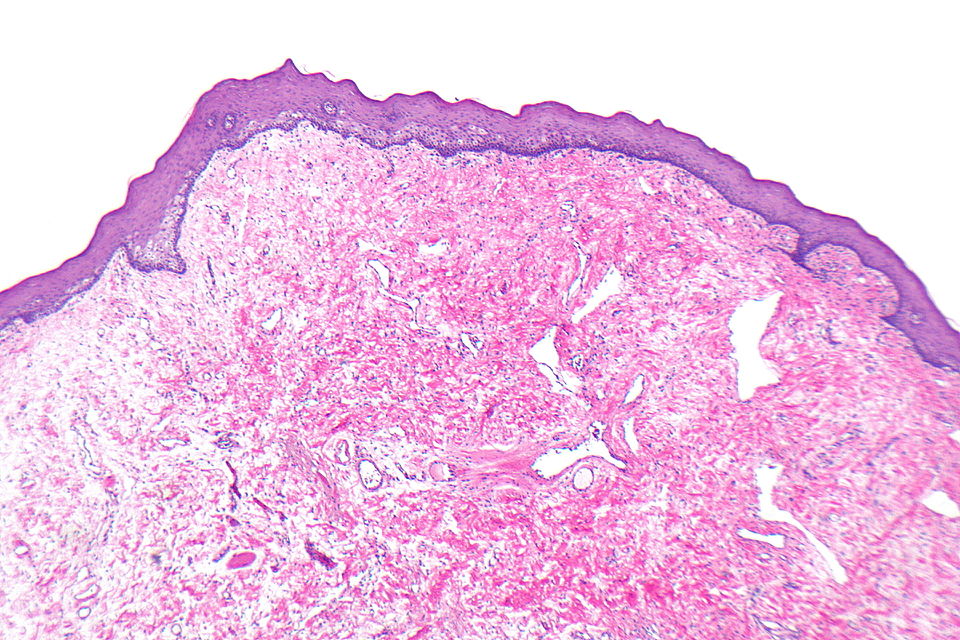
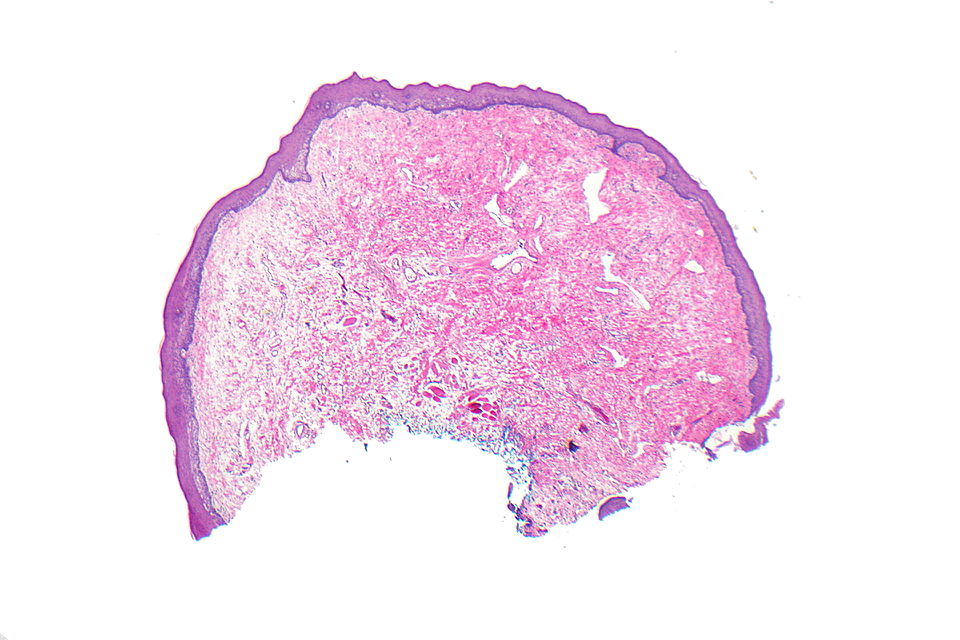
Oral Fibroma (Focal fibrous hyperplasia, Peripheral fibroma, Peripheral ossifying fibroma, Fibroid epulis, Fibroepithelial polyp, Irritation fibroma, Traumatic fibroma)
The oral fibroma is also referred to as irritation fibroma. The majority of fibromas represent reactive focal fibrous hyperplasia due to trauma or local irritation.[1]
Associated Conditions
Multiple oral fibromas may be seen in Cowden disease.
Location
A fibroma may occur at any oral site, but it is seen most often on the buccal mucosa along the plane of occlusion of the maxillary and mandibular teeth.
Pathogenesis
The oral fibroma is a common oral lesion that arises due to trauma or chronic irritation. The fibroma is considered to be a reactive lesion. Oral fibroma represents a response of connective tissue cells to chronic irritation. When trauma occurs, the tissues of the oral cavity react and an irrepressible repair process is seen. As a result, an overabundance of fibrous connective tissue is produced and the formation of a nodule or mass.
Gross Pathology
On gross pathology, a round-to-ovoid polypoid lesion, smooth-surfaced, and firm sessile or pedunculated mass, 1mm to 2 cm in diameter in size, hyperkeratotic or ulcerated surface, owing to repeated trauma are characteristic findings of oral fibroma.
Microscopic Pathology
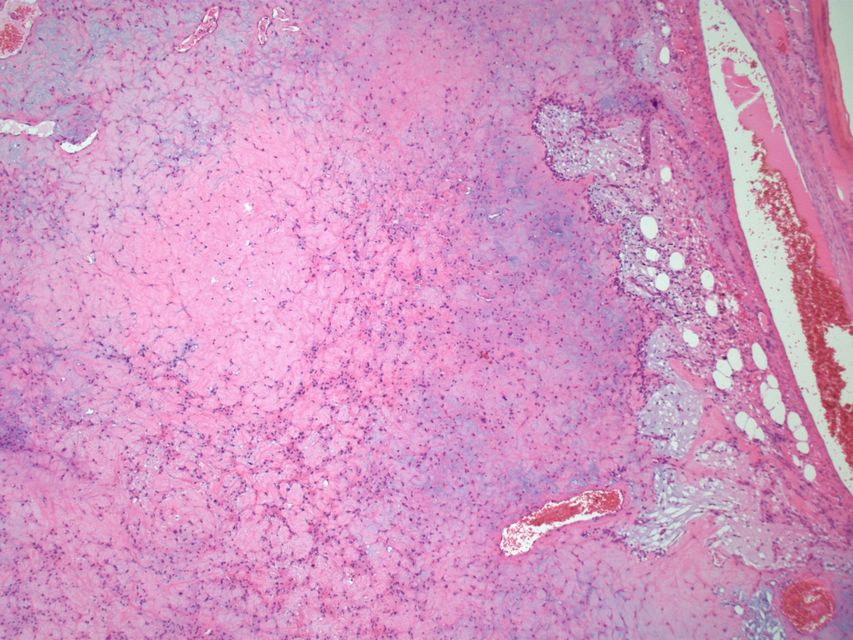
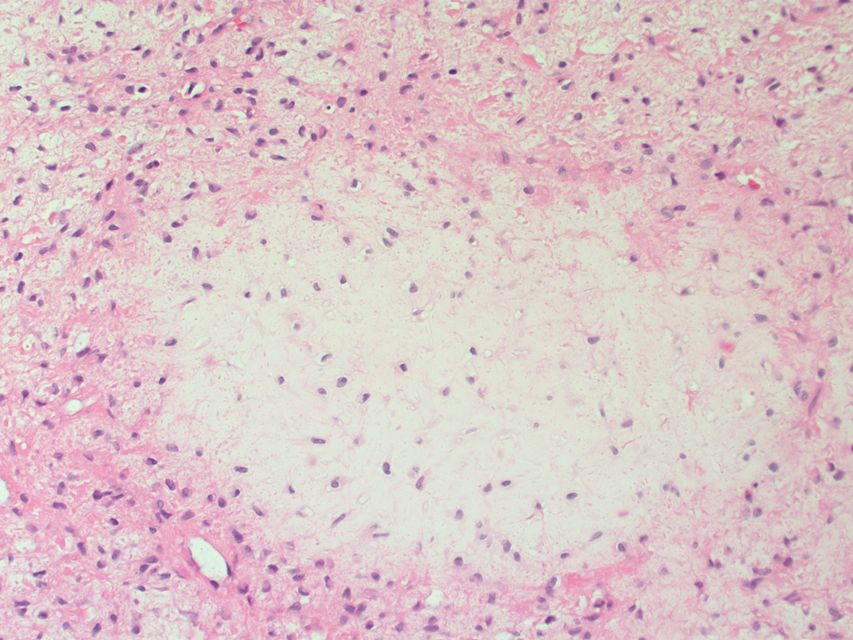
Following features are noted on microscopic histopathological analysis of oral fibroma:[3][4]
- Histologically similar to fibrous papule
- Fibrous stroma is a key feature
- Collagen bundles may be present
- Vessels may be prominent
- Overlying squamous mucosa is benign
- Hyperkeratosis may be present
- Focal ulceration may be present
-
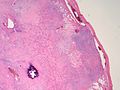
Histopathology specimen of a oral fibroma low magnification
-
Histopathology specimen of a oral fibroma very low magnification
-
Histopathology specimen of a oral fibroma intermediate magnification
Chondromyxoid Fibroma
Location
- Approximately 90% of chondromyxoid fibromas involve the lower extremity. The proximal tibia metaphysis is the most common location, followed by the distal femoral metaphysis.
- In younger patients, long bones are involved much more frequently than are other bones. Flat bone involvement may rarely be seen in older patients.
- It is a slow-growing, locally destructive tumor that usually affects the metaphyseal region of long bones. Involvement of the skull base and orbit is extremely rare.[5]
Gross Pathology
On gross pathology, well circumscribed, metaphyseal lesion, and fragments of white-grey rubbery tissue are characteristic findings of chondromyxoid fibroma.
Microscopic Pathology
Following features are noted on microscopic histopathological analysis of chondromyxoid fibroma:
- Spindle cells or stellate cells in a myxoid or chondroid stroma
- Lobules with hypocellular centers and hypercellular peripheries
- Giant cells in the hypercellular periphery
- Scattered calcifications.
- No true hyaline cartilage formation is seen
- No mitotic activity is seen
Genetics
The majority of cases of chondromyxoid fibroma are characterised by GRM1 gene fusion or promoter swapping. It can be associated with a translocation at t(1;5)(p13;p13)
-
Histopathology specimen of a chondromyxoid fibroma showing stellate cells in myxoid stroma
-
Histopathology specimen of a chondromyxoid fibroma showing lobule with calcification
-
Histopathology specimen of a chondromyxoid fibroma showing stellate cells in myxohyaline stroma
-
Histopathology specimen of a chondromyxoid fibroma showing stellate cells in myxoid stroma
-
Histopathology specimen of a chondromyxoid fibroma showing focus of amorphous calcification
-
Histopathology specimen of a chondromyxoid fibroma showing foci of amorphous calcification
-
Histopathology specimen of a chondromyxoid fibroma showing stellate cells in myxohyaline stroma
-
Histopathology specimen of a chondromyxoid fibroma showing Lobule with a darker (hypercellular) periphery and lighter (hypocellular) center. (SKB)
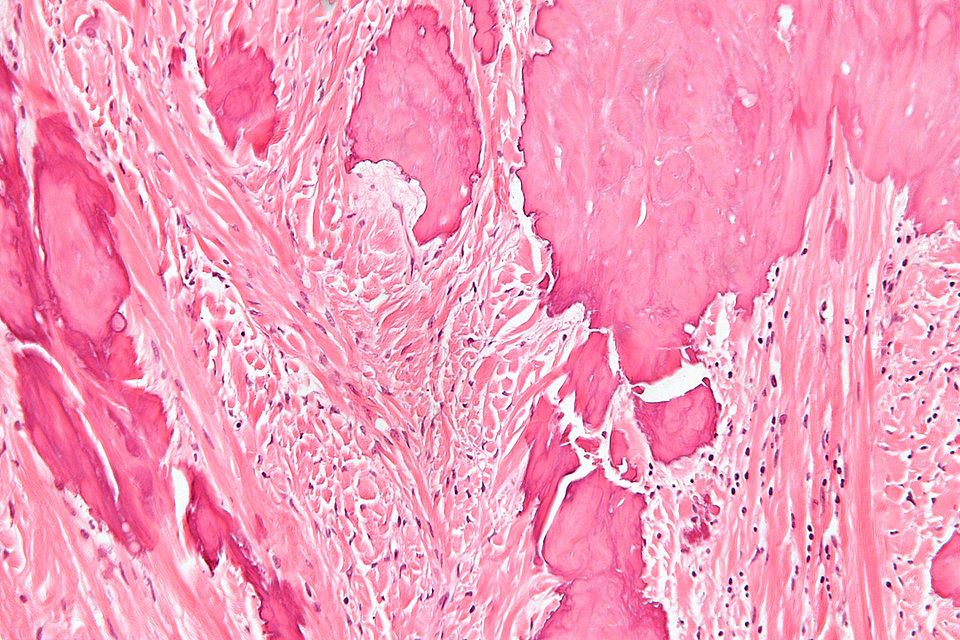
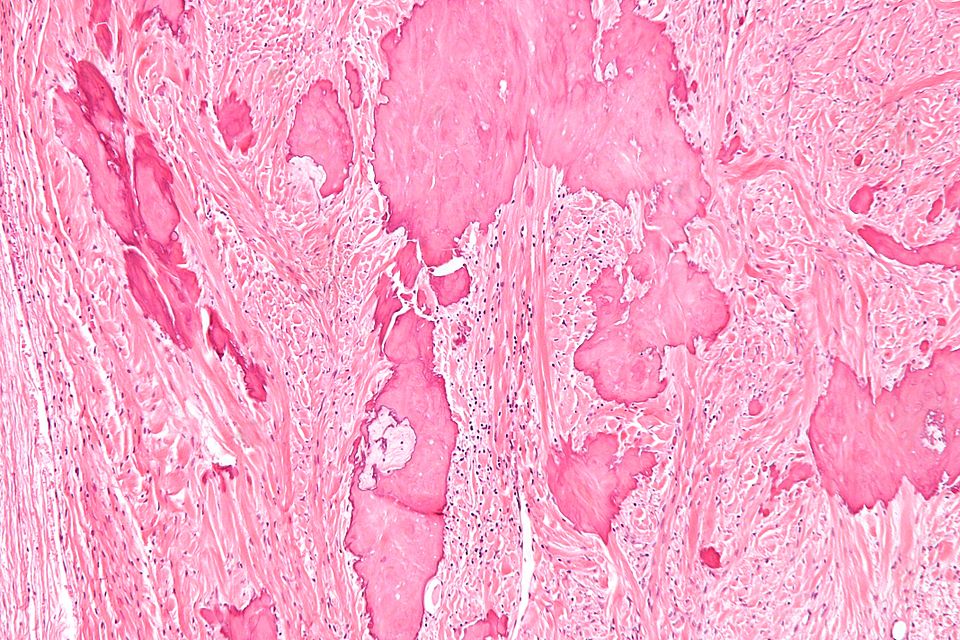
Ossifying Fibroma (Osteofibrous dysplasia, Congenital osteitis fibrosa)
Gross Pathology
On gross pathology, discrete mass that is well delineated from surrounding bone, tan-white, rubbery cut surface, firm to gritty and no encapsulation are characteristic findings of ossifying fibroma.
Microscopic Pathology
Following features are noted on microscopic histopathological analysis of ossifying fibroma:
- They comprise of haphazardly distributed lamellated bony spicules on a background of fibrous stroma.
- Despite being benign, they can be locally aggressive.
- Immunohistochemical staining of lesions shows positive keratin cells in the majority of the cases.
- The lesion has a zonal architecture with a center of immature bone surrounded by more mature lamellar bone.
- The central spicules of woven bony trabeculae are lined by a layer of osteoblasts. The background is a loose and storiform fibrous tissue.
Location
- Lower extremity
- Tibia: It is the most frequent site (approximately 90% of the time); there is a predilection for the cortex of the tibial diaphysis.
- Femur: It occurs in a diaphyseal location
Immunohistochemistry
- Ossifying fibromas are keratin positive - isolated cells accepted by some.
- It is osteonectin, neurofibromin, and S-100 protein positive.
Associated Conditions
- It is associated with clonal chromosomal abnormalities such as trisomies of chromosomes 7, 8, 12,21, and/or 22
Pathogenesis
- Ossifying fibroma may be either a clonal neoplastic lesion or a developmental dysplasia.
Associated Conditions
- It be related to adamantinoma
Non-ossifying Fibroma (nonossifying fibroma, fibrous cortical defect, fibrous metaphyseal defect, fibroxanthoma of bone, histiocytic fibrous defect, histiocytic xanthogranuloma)
Gross Pathology
On gross pathology, fleshy, fibrous, yellow or tan-brown lesion with variable areas of haemorrhage are characteristic findings of non-ossifying fibroma.
Microscopic Pathology
The following features are seen on microscopic histopathological analysis of non-ossifying fibroma:
- Spindle cells without cytologic atypia are arranged in a storiform pattern.
- Scattered chronic inflammatory cells and benign giant cells.
- Foam cells and hemosiderin deposition are present.
- Mitoses are seen but cytologic atypia is absent.
-
Metaphyseal fibrous defect - high magnification.
-
Metaphyseal fibrous defect - intermediate magnification.
Location
- Metaphysis of distal femur or proximal tibia (80%)
- Cortical
- Metaphysis
- Long bone
- Eccentric location
Associated Conditions
- Neurofibromatosis-type 1 (NF1)
- Fibrous dysplasia
- Jaffe-Campanacci syndrome
Genetics
There are reports of non-ossifying fibroma (NOF) in the long bones with clonal rearrangements in chromosomes 1, 3, 4, 11, and 14. These clonal chromosomal changes may suggest genetic events associated with tumorigenesis in the reported NOFs. However, no studies have reported clonal rearrangements in NOF of the mandible.[6]
Desmoplastic Fibroma
Microscopic Pathology
The following features are seen on microscopic histopathological analysis of desmoplastic fibroma:
- Lamellar bone
- Fibrotic marrow space has following features:
- Collagen
- Low cellularity
- Spindle cells without significant atypia
- On histopathology, desmoplastic fibromas are identical to soft tissue desmoid tumors, with abundant collagenous stroma and little cellularity or pleomorphism.
- The main cell types that are seen include the following:
- Fibroblasts
- Myofibroblasts
- Undifferentiated mesenchymal cells
Location
- Desmoplastic fibromas can affect any bone, but the majority of cases occur in the mandible (22%) and the metaphysis of long bones (56%).
- The pelvis accounts for 12% of cases.[7]
Immunohistochemistry
- Strong positive for the adhesion protein β-Catenin
- Weak positive labelling for S-100
- No positive stain for the proliferation marker Ki67
Pleural Fibroma (Solitary fibrous tumor (SFT), also known as fibrous tumor of the pleura)
Microscopic Pathology
The following features are seen on microscopic histopathological analysis of pleural fibroma:
- Pleural fibromas are composed of irregularly arranged fascicles comprising of spindle cells with collagen separation.
- They originate from submesothelial mesenchymal cells.
- Myxoid or cystic degeneration can occur.
Location
- Approximately 80% of pleural fibromas arise from the visceral pleura, with the remainder arising from the parietal pleura. About 80% of pleural fibromas originate in the visceral pleura, while 20% arise from parietal pleura.
- There may be a predilection towards the mid to lower zones of the chest.
Associated Conditions
- Some pleural fibromas are associated with the paraneoplastic Doege–Potter syndrome, which is caused by tumor production of IGF-2.
- Hypoglycaemia: 2-4% 6, thought to be due to production of IGF-2
- Hypertrophic pulmonary osteoarthropathy (HPOA): ~20% 6: thought to be due to abnormal production of hyaluronic acid
Genetics
Recurrent somatic fusions of the two genes, NGFI-A–binding protein 2 (NAB2) and STAT6, located at chromosomal region 12q13, have been identified in pleural fibromas.
Cardiac Fibroma
Microscopic Pathology
The following features are seen on microscopic histopathological analysis of cardiac fibromas:
- Cardiac fibromas are well defined, solitary, intramyocardial lesions with smooth margins and usually large, with a mean diameter of ~5 cm and may obliterate the ventricular cavity.
- Unlike many primary cardiac tumours, fibromas usually have no foci of cystic change, hemorrhage, or necrosis. Calcification is a common findings.
Associated Conditions
There is an increased prevalence of cardiac fibromas in Gorlin syndrome.
Location
The majority of cardiac fibromas arise in the ventricular septum and left ventricular free wall.
Cemento-ossifying Fibroma (Peripheral ossifying fibroma, calcifying fibrous epulis, peripheral fibroma with calcification)
When the tumor occurs in children, it is called the juvenile aggressive cemento-ossifying fibroma.
Location
Most often, it is located in the gingival papilla between adjacent teeth. The maxillary gingiva is involved more often than the mandibular gingiva; the anterior region is usually affected. Occasionally mobility and/or migration of adjacent teeth is observed. The mandible is a common site for central cemento-ossifying fibromas; the maxillary sinus is an unusual site for central cemento-ossifying fibromas.
Gross Pathology
On gross pathology, solid, sessile or pedunculated mass, which is often ulcerated, and generally has a diameter of less than 2 cm are characteristic findings of cemento-ossifying fibroma.
Microscopic Pathology
The following features are seen on microscopic histopathological analysis of cemento-ossifying fibroma:
- Cemento -ossifying tumours are composed of fibrous tissue, calcified tissue resembling bone and/or cementum.
- The bone-like component is predominant reminiscent of woven bone and is found in more 'mature' lesions.
- In some instances, this entity has been divided into cementifying fibroma and ossifying fibroma depending on the relative amounts of the tumor's constituent tissues. *However, in majority of cases both features are present, warranting the generic term cemento-ossifying fibromas.
- Surface ulceration is common
Pathogenesis
- Cemento-ossifying tumors are thought to arise from the periodontal ligament, accounting for the usual vicinity to teeth.
Renal Medullary Fibroma
Gross pathology
On gross pathology, small, and white well circumscribed nodule in medulla typically less than 3mm are characteristic findings of renal medullary fibroma.
Microscopic Pathology
The following features are seen on microscopic histopathological analysis of renal medullary fibroma:
- Small polygonal/stellate cells
- Abundant loose/myxoid stroma
- Entrapped renal tubules may be present
Ameloblastic Fibroma
Microscopic Pathology
The following features are seen on microscopic histopathological analysis of ameloblastic fibroma:
- Palisaded nuclei
- Fibrous stroma
Desmoplastic Fibroblastoma (Collagenous Fibroma)
Location
Classically found in the shoulder region.
Microscopic Pathology
The following features are seen on microscopic histopathological analysis of desmoplastic fibroblastoma:
- Spindle cells or stellate cells without nuclear atypia
- Acellular stroma with abundant collagen
- Myxoid areas may be present
- Mitoses is rare
Immunohistochemistry
The following findings are noted on immunohistochemistry of desmoplastic fibroblastoma:
- Beta-catenin -ve
- Positive in desmoid-type fibromatosis
- Desmin negative
- S-100 negative
- CD34 negative
- MSA positive
- Alpha-SMA positive
Pathogenesis
- FOSL1 gene is involved in the pathogenesis of desmoplastic fibroblastoma, llq 12 breakpoint described as being characteristic.
Elastofibroma
Location
- The elastofibroma is most often seen in the subscapular region.
Gross Pathology
On gross pathology, ill defined, nonencapsulated, rubbery, firm, white lesion with interspersed fat and with moderate demarcation to surrounding tissue are characteristic findings of elastofibroma. The tumors can be quite large (up to 20 cm), although most are around 5 cm.
Microscopic Pathology
- On microscopic histopathological analysis, thick bundles of collagen and elastin fibres are characteristic findings of elastofibroma.
- On microscopic histopathological analysis, there is an admixture of heavy dense bands of collagenous tissue dissected by fat and abnormal elastic fibers. The elastic fibers are often quite large and are easily identified. The elastic fibers are coarse, thick, and darkly eosinophilic, often fragmented into globules, creating a "string of pearls" or "pipe cleaner" appearance. Because of degeneration, the elastic fibers will appear as globules with a serrated or "prickled" edge.
Immunohistochemistry
The elastic fibers will be visible on a Weigert or von Gieson elastic stains.
Genetics
- There are alterations of short arm of chromosome 1
- Multifocality may suggest systemic enzymatic defect, resulting in abnormal elastogenesis
- Repeated trauma or friction seems unlikely, but is still a possibility
Sclerotic Fibroma
Associated Conditions
- Cowden syndrome
Location
- It occurs most commonly in the skin and may be solitary or multifocal.
- Both sporadic sclerotic fibromas and those associated with the Cowden syndrome have been described in the oral cavity, mainly in the labial and buccal mucosa.
The sclerotic fibroma was first described as a component of Cowden syndrome.
Microscopic Pathology
On microscopic histopathological analysis, well-delineated but unencapsulated mass of densely collagenized, hypocellular fibrous tissue with a storiform pattern, and prominent clefts between collagen bundles are characteristic findings of elastofibroma.
Immunohistochemistry
- CD34
- Vimentin positive
Uterine Fibroma (uterine leiomyoma, myoma, fibromyoma, fibroleiomyoma, uterine fibroids
Location
Fibroids may have a number of locations within or external to the uterus:
- Intra-uterine
- Intramural leiomyoma: Intramural fibroids are located within the wall of the uterus and are the most common type
- Subserosal leiomyoma:Subserosal fibroids are located underneath the peritoneal surface of the uterus and can become very large. They can also grow out in a papillary manner to become pedunculated fibroids. These pedunculated growths can actually detach from the uterus to become a parasitic leiomyoma.
- Submucosal leiomyoma: Submucosal fibroids are located in the muscle beneath the endometrium of the uterus and distort the uterine cavity; A pedunculated lesion within the cavity is termed an intracavitary fibroid and can be passed through the cervix. Submucosal leiomyomas are least common (10-15%)
- Extra-uterine
- Broad ligament leiomyoma
- Cervical leiomyoma
- Parasitic leiomyoma
- Diffuse uterine leiomyomatosis
- Cervical fibroids are located in the wall of the cervix (neck of the uterus). Rarely, fibroids are found in the supporting structures (round ligament, broad ligament, or uterosacral ligament) of the uterus that also contain smooth muscle tissue.
Gross Pathology
On gross pathology, round, well circumscribed (but not encapsulated), solid nodules that are white or tan, and show whorled appearance on histological section are characteristic findings of uterine fibroma.
Microscopic Pathology
On microscopic histopathological analysis of uterine fibroma, the following features are seen:
- Spindle cells arranged in fascicles.
- Fascicular appearance: adjacent groups of cells have their long axis perpendicular to one another.
- Whorled arrangement of cells.
- These cells are uniform in size and shape, with scarce mitoses.
- There are three benign variants: bizarre (atypical); cellular; and mitotically active.
- The appearance of prominent nucleoli with perinucleolar halos should alert the pathologist to investigate the possibility of the extremely rare hereditary leiomyomatosis and renal cell cancer (Reed) syndrome.
Pathogenesis
- It is believed that estrogen and progesterone have a mitogenic effect on leiomyoma cells and also act by influencing (directly and indirectly) a large number of growth factors, cytokines and apoptotic factors as well as other hormones.
- Furthermore, the actions of estrogen and progesterone are modulated by the cross-talk between estrogen, progesterone and prolactin signalling which controls the expression of the respective nuclear receptors.
- It is believed that estrogen promotes growth by up-regulating IGF-1, EGFR, TGF-beta1, TGF-beta3 and PDGF, and promotes aberrant survival of leiomyoma cells by down-regulating p53, increasing expression of the anti-apoptotic factor PCP4 and antagonizing PPAR-gamma signalling. Progesterone is thought to promote the growth of leiomyoma through up-regulating EGF, TGF-beta1 and TGF-beta3, and promotes survival through up-regulating Bcl-2 expression and down-regulating TNF-alpha. Progesterone is believed to counteract growth by downregulating IGF-1.
- Expression of transforming growth interacting factor (TGIF) is increased in leiomyoma compared with myometrium. TGIF is a potential repressor of TGF-β pathways in myometrial cells.
- Aromatase and 17beta-hydroxysteroid dehydrogenase are aberrantly expressed in fibroids, indicating that fibroids can convert circulating androstenedione into estradiol.
- First degree relatives have a 2.5-fold risk, and nearly 6-fold risk when considering early onset cases. Monozygotic twins have double concordance rate for hysterectomy compared to dizygotic twins.
- Expansion of uterine fibroids is by a slow rate of cell proliferation combined with the production of copious amounts of extracellular matrix.
- A small population of the cells in an uterine fibroid have properties of stem cells or progenitor cells, and contribute significantly to ovarian steroid-dependent growth of fibroids. These stem-progenitor cells are deficient in estrogen receptor α and progesterone receptor and instead rely on substantially higher levels of these receptors in surrounding differentiated cells to mediate estrogen and progesterone actions via paracrine signalling.
Genetics
- Fibroids are monoclonal tumors and approximately 40 to 50% show karyotypically detectable chromosomal abnormalities.
- When multiple fibroids are present they frequently have unrelated genetic defects. Specific mutations of the MED12 protein have been noted in 70 percent of fibroids.
- If a mother had fibroids, risk in the daughter is about three times higher than average. Researchers have found that only a few specific genes or cytogenetic deviations are associated with uterine leiomyomas. An association with fatty acid synthase has been reported.
Associated Conditions
A syndrome (Reed's syndrome) that causes uterine leiomyomata along with cutaneous leiomyomata and renal cell cancer has been reported. This is associated with a mutation in the gene that produces the enzyme fumarate hydratase, located on the long arm of chromosome 1 (1q42.3-43). Inheritance is autosomal dominant.
Immunohistochemistry
- CD10 positive
- SMA positive
- Desmin positive
- H-caldesmon positive
- p16 negative
- Ki-67 negative
Giant cell fibroma
Giant-cell fibroma is a type of fibroma not associated with trauma or irritation. It is a localized reactive proliferation of fibrous connective tissue. Giant cell fibroma is the oral counterpart of fibrous papule of the nose.
Location
The most common sites for giant cell fibroma are the mandibular gingiva, followed by the maxillary gingiva, the tongue, and the palate.
Gross Pathology
On gross pathology, sessile or pedunculated nodule that is smaller than 1cm in diameter are characteristic findings of giant cell fibroma. Often, it has a bosselated or somewhat papillary surface.
Microscopic Pathology
The following features are seen on microscopic histopathological analysis of giant cell fibroma:
- An unencapsulated mass of fibrous connective tissue that contains numerous characteristic large, plump, stellate and spindle-shaped fibroblasts, some of which are multinucleated. These stellate and spindle shaped cells are easily observed in the peripheral areas of the lesion, while the more central areas contain typical fusiform fibroblasts.
- The surface epithelium is often atrophic and corrugated.
Peripheral Odontogenic Fibroma
Pathogenesis
A peripheral odontogenic fibroma is a an uncommon neoplasm that is believed to arise from odontogenic epithelial rests in the periodontal ligament or the attached gingiva itself. Peripheral odontogenic fibroma is considered to be the extraosseous counterpart of the central odontogenic fibroma of the World Health Organization type.
Location
Peripheral odontogenic fibroma is commonly seen in mandible than maxilla. It is most often seen on the mandibular buccal or labial aspect.
Gross Pathology
On gross pathology, firm, slowly growing, sessile, and nodular growth of the gingiva are characteristic findings of peripheral odontogenic fibroma.
Microscopic Pathology
On microscopic histopathological analysis of peripheral odontogenic fibroma, the following features are seen:
- An unencapsulated mass of interwoven cellular fibrous connective tissue that contains scattered nests or strands of odontogenic epithelium
- It consists of cellular fibrous connective tissue parenchyma with non neoplastic islands, strands of columnar or cuboidal odontogenic epithelium.
- Myxoid foci, osteoid, cementoid, or dystrophic calcifications are sometimes seen.
- Generally, the surface is not ulcerated.
References
- ↑ 1.0 1.1 Fibroma. Libre pathology(2015) http://librepathology.org/wiki/Fibroma Accessed on March 12, 2016
- ↑ Fibroma. Radiopedia(2015) http://radiopaedia.org/search?utf8=%E2%9C%93&q=fibroma&scope=all Accessed on March 12, 2016
- ↑ Barker DS, Lucas RB (1967). "Localised fibrous overgrowths of the oral mucosa". Br J Oral Surg. 5 (2): 86–92. PMID 5235090.
- ↑ Esmeili T, Lozada-Nur F, Epstein J (2005). "Common benign oral soft tissue masses". Dent Clin North Am. 49 (1): 223–40, x. doi:10.1016/j.cden.2004.07.001. PMID 15567370.
- ↑ Khalatbari, Mahmoud; Hamidi, Mehrdokht; Moharamzad, Yashar (2012). "Chondromyxoid Fibroma of the Anterior Skull Base Invading the Orbit in a Pediatric Patient: Case Report and Review of the Literature". Neuropediatrics. 43 (03): 140–145. doi:10.1055/s-0032-1307460. ISSN 0174-304X.
- ↑ Bowers LM, Cohen DM, Bhattacharyya I, Pettigrew JC, Stavropoulos MF (2013). "The non-ossifying fibroma: a case report and review of the literature". Head Neck Pathol. 7 (2): 203–10. doi:10.1007/s12105-012-0399-7. PMC 3642261. PMID 23008139.
- ↑ Nedopil A, Raab P, Rudert M (2013). "Desmoplastic fibroma: a case report with three years of clinical and radiographic observation and review of the literature". Open Orthop J. 8: 40–6. doi:10.2174/1874325001307010040. PMC 3583030. PMID 23459513.