Evolocumab: Difference between revisions
Sergekorjian (talk | contribs) |
No edit summary |
||
| (16 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | |||
|authorTag={{SCh}} | |||
<!--Overview--> | |||
|genericName=Evolocumab | |||
|aOrAn=a | |||
|drugClass=[[PCSK9]] (proprotein convertase subtilisin kexin type9) inhibitor antibody | |||
|indicationType=treatment | |||
|indication=[[Primary Hyperlipidemia]], Homozygous [[Familial Hypercholesterolemia]], Heterozygous [[Familial Hypercholesterolemia]], or Clinical [[atherosclerotic cardiovascular disease]] (CVD). | |||
= | |adverseReactions=[[Nasopharyngitis]], [[upper respiratory tract infection]], [[Influenza]], [[back pain]], and [[injection site reactions]]. | ||
<!--Adult Indications and Dosage--> | |||
==== | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult===== Primary Hyperlipidemia ==== | |||
* Evolocumab is indicated as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous [[familial hypercholesterolemia]] (HeFH) or clinical [[atherosclerotic cardiovascular disease]] (CVD), who require additional lowering of [[low density lipoprotein]] cholesterol (LDL-C). | |||
==== | ====Homozygous Familial Hypercholesterolemia ==== | ||
* Evolocumab is indicated as an adjunct to diet and other [[LDL]]-lowering therapies (e.g., [[statins]], [[ezetimibe]], LDL apheresis) for the treatment of patients with homozygous [[familial hypercholesterolemia]] (HoFH) who require additional lowering of LDL-C. | |||
=== Dosage forms and strengths === | |||
* Evolocumab is a sterile, clear to opalescent, colorless to pale yellow solution available as follows: | |||
** Injection: 140 mg/mL solution in a single-use profiled syringe. | |||
** Injection: 140 mg/mL solution in a single-use prefilled SureClick® auto injector. | |||
** Injection: 420 mg/3.5 mL solution in a single-use PushtronexTM system (on-body infusor with prefilled cartridge). | |||
<!--Contraindications--> | |||
|contraindications=* Evolocumab is contraindicated in patients with a history of a serious [[hypersensitivity]] reaction to Evolocumab. | |||
==== | <!--Warnings--> | ||
|warnings===== Allergic Reactions ==== | |||
* Hypersensitivity reactions (e.g., [[rash]], [[urticaria]]) have been reported in patients treated with Evolocumab, including some that led to discontinuation of therapy. | |||
* If signs or symptoms of serious allergic reactions occur, discontinue treatment with Evolocumab, treat according to the standard of care, and monitor until signs and symptoms resolve. | |||
<!--Adverse Reactions--> | |||
==== | <!--Clinical Trials Experience--> | ||
|clinicalTrials==== Clinical Trials Experience === | |||
* Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. | |||
* Adverse Reactions in Patients with Primary Hyperlipidemia and in Patients with Heterozygous Familial Hypercholesterolemia | |||
:* Evolocumab is not indicated for use in patients without [[familial hypercholesterolemia]] or atherosclerotic CVD. | |||
:* The data described below reflect exposure to Evolocumab in 8 placebo-controlled trials that included 2651 patients treated with Evolocumab, including 557 exposed for 6 months and 515 exposed for 1 year (median treatment duration of 12 weeks). | |||
:* The mean age of the population was 57 years, 49% of the population were women, 85% White, 6% Black, 8% Asians, and 2% other races. | |||
* Adverse Reactions in a 52-Week Controlled Trial | |||
:* In a 52-week, double-blind, randomized, placebo-controlled trial (Study 2), 599 patients received 420 mg of Evolocumab [[subcutaneously]] once monthly. | |||
:* The mean age was 56 years (range: 22 to 75 years), 23% were older than 65 years, 52% women, 80% White, 8% Black, 6% Asian, and 6% Hispanic. | |||
:* Adverse reactions reported in at least 3% of Evolocumab-treated patients, and more frequently than in placebo-treated patients in Study 2, are shown in Table 1. | |||
:* Adverse reactions led to discontinuation of treatment in 2.2% of Evolocumab-treated patients and 1% of placebo-treated patients. | |||
:* The most common adverse reaction that led to Evolocumab treatment discontinuation and occurred at a rate greater than placebo was [[myalgia]] (0.3% versus 0% for Evolocumab and placebo, respectively). | |||
[[File:RepathaADR.png|thumb|center|This image is provided by the National Library of Medicine]] | |||
† includes erythema, pain, bruising. | |||
* Adverse Reactions in Seven Pooled 12-Week Controlled Trials | |||
:* In seven pooled 12-week, double-blind, randomized, placebo-controlled trials, 993 patients received 140 mg of Evolocumab subcutaneously every 2 weeks and 1059 patients received 420 mg of Evolocumab subcutaneously monthly. | |||
:* The mean age was 57 years (range: 18 to 80 years), 29% were older than 65 years, 49% women, 85% White, 5% Black, 9% Asian, and 5% Hispanic. | |||
:* Adverse reactions reported in at least 1% of Evolocumab-treated patients, and more frequently than in placebo-treated patients, are shown in Table 2. | |||
[[File:REPATHAADR.png|thumb|center|This image is provided by the National Library of Medicine]] | |||
* Adverse Reactions in Eight Pooled Controlled Trials (Seven 12-Week Trials and One 52-Week Trial) | |||
:* The adverse reactions described below are from a pool of the 52-week trial (Study 2) and seven 12-week trials. | |||
:* The mean and median exposure durations of Evolocumab in this pool of eight trials were 20 weeks and 12 weeks, respectively. | |||
* Local Injection Site Reactions | |||
:* Injection site reactions occurred in 3.2% and 3.0% of Evolocumab-treated and placebo-treated patients, respectively. | |||
:* The most common injection site reactions were [[erythema]], [[pain]], and [[bruising]]. | |||
:* The proportions of patients who discontinued treatment due to local injection site reactions in Evolocumab-treated patients and placebo-treated patients were 0.1% and 0%, respectively. | |||
* Allergic Reactions | |||
:* Allergic reactions occurred in 5.1% and 4.7% of Evolocumab-treated and placebo-treated patients, respectively. | |||
:* The most common allergic reactions were [[rash]] (1.0% versus 0.5% for Evolocumab and placebo, respectively), [[eczema]] (0.4% versus 0.2%), [[erythema]] (0.4% versus 0.2%), and [[urticaria]] (0.4% versus 0.1%). | |||
* Neurocognitive Events | |||
:* In placebo-controlled trials, neurocognitive events were reported in less than or equal to 0.2% in Evolocumab-treated and placebo-treated patients. | |||
* Low LDL-C Levels | |||
:* In a pool of placebo- and active-controlled trials, as well as open-label extension studies that followed them, a total of 1988 patients treated with Evolocumab had at least one LDL-C value < 25 mg/dL. | |||
:* Changes to background lipid-altering therapy were not made in response to low LDL-C values, and Evolocumab dosing was not modified or interrupted on this basis. | |||
:* Although adverse consequences of very low LDL-C were not identified in these trials, the long-term effects of very low levels of LDL-C induced by Evolocumab are unknown. | |||
* Musculoskeletal Events | |||
:* Musculoskeletal adverse reactions were reported in 14.3% of Evolocumab-treated patients and 12.8% of placebo-treated patients. | |||
:* The most common adverse reactions that occurred at a rate greater than placebo were [[back pain]] (3.2% versus 2.9% for Evolocumab and placebo, respectively), [[arthralgia]] (2.3% versus 2.2%), and [[myalgia]] (2.0% versus 1.8%). | |||
* Adverse Reactions in Patients with Homozygous Familial Hypercholesterolemia | |||
:* In a 12-week, double-blind, randomized, placebo-controlled trial of 49 patients with HoFH (Study 4), 33 patients received 420 mg of Evolocumab subcutaneously once monthly [see Clinical Studies (14.3)]. | |||
:* The mean age was 31 years (range: 13 to 57 years), 49% were women, 90% White, 4% Asian, and 6% other. | |||
:* The adverse reactions that occurred in at least two (6.1%) Evolocumab-treated patients, and more frequently than in placebo-treated patients, included: | |||
** [[Upper respiratory tract infection]](9.1% versus 6.3%). | |||
** [[Influenza]] (9.1% versus 0%). | |||
** [[Gastroenteritis]] (6.1% versus 0%). | |||
** [[Nasopharyngitis]] (6.1% versus 0%). | |||
==== Immunogenicity ==== | |||
* As with all therapeutic proteins, there is potential for immunogenicity. | |||
* The immunogenicity of Evolocumab has been evaluated using an electrochemiluminescent bridging screening immunoassay for the detection of binding anti-drug antibodies. | |||
* For patients whose sera tested positive in the screening immunoassay, an in vitro biological assay was performed to detect neutralizing antibodies. | |||
* In a pool of placebo- and active-controlled clinical trials, 0.1% of patients treated with at least one dose of Evolocumab tested positive for binding antibody development. | |||
* Patients whose sera tested positive for binding antibodies were further evaluated for neutralizing antibodies; none of the patients tested positive for neutralizing antibodies. | |||
* There was no evidence that the presence of anti-drug binding antibodies impacted the pharmacokinetic profile, clinical response, or safety of Evolocumab, but the long-term consequences of continuing Evolocumab treatment in the presence of anti-drug binding antibodies are unknown. | |||
* The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. | |||
* Additionally, the observed incidence of antibody positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. | |||
* For these reasons, comparison of the incidence of antibodies to Evolocumab with the incidence of antibodies to other products may be misleading. | |||
<!--Use in Specific Populations--> | |||
|useInPregnancyFDA==== Pregnancy === | |||
* Risk Summary | |||
:* There are no data available on use of Evolocumab in pregnant women to inform a drug-associated risk. | |||
:* In animal reproduction studies, there were no effects on pregnancy or neonatal/infant development when monkeys were subcutaneously administered [[evolocumab]] from organogenesis through parturition at dose exposures up to 12 times the exposure at the maximum recommended human dose of 420 mg every month. | |||
:* In a similar study with another drug in the [[PCSK9 inhibitor]] antibody class, humoral immune suppression was observed in infant monkeys exposed to that drug in utero at all doses. | |||
:* The exposures where immune suppression occurred in infant monkeys were greater than those expected clinically. | |||
:* No assessment for immune suppression was conducted with [[evolocumab]] in infant monkeys. | |||
:* Measurable evolocumab serum concentrations were observed in the infant monkeys at birth at comparable levels to maternal serum, indicating that evolocumab, like other [[IgG antibodies]], crosses the placental barrier. | |||
:* FDA’s experience with monoclonal antibodies in humans indicates that they are unlikely to cross the placenta in the first trimester; however, they are likely to cross the placenta in increasing amounts in the second and third trimester. | |||
:* Consider the benefits and risks of Evolocumab and possible risks to the fetus before prescribing Evolocumab to pregnant women. | |||
:* In the U.S. general population, the estimated background risk of major birth defects and [[miscarriage]] in clinically recognized pregnancies is 2-4% and 15-20%, respectively. | |||
* '''Data''' | |||
* Animal Data | |||
:* In cynomolgus monkeys, no effects on embryo-fetal or postnatal development (up to 6 months of age) were observed when evolocumab was dosed during organogenesis to parturition at 50 mg/kg once every 2 weeks by the subcutaneous route at exposures 30- and 12-fold the recommended human doses of 140 mg every 2 weeks and 420 mg once monthly, respectively, based on plasma AUC. | |||
:* No test of [[humoral immunity]] in infant monkeys was conducted with evolocumab. | |||
|useInNursing==== Lactation === | |||
* Risk Summary | |||
:* There is no information regarding the presence of evolocumab in human milk, the effects on the breastfed infant, or the effects on milk production. | |||
:* The development and health benefits of [[breastfeeding]] should be considered along with the mother’s clinical need for Evolocumab and any potential adverse effects on the breastfed infant from Evolocumab or from the underlying maternal condition. | |||
:* Human [[IgG]] is present in human milk, but published data suggest that breast milk antibodies do not enter the neonatal and infant circulation in substantial amounts. | |||
|useInPed==== Pediatric Use === | |||
* The safety and effectiveness of Evolocumab in combination with diet and other LDL-C-lowering therapies in adolescents with [[HoFH]] who require additional lowering of LDL-C were established based on data from a 12-week, placebo-controlled trial that included 10 adolescents (ages 13 to 17 years old) with [[HoFH]]. | |||
* In this trial, 7 adolescents received Evolocumab 420 mg subcutaneously once monthly and 3 adolescents received placebo. | |||
* The effect of Evolocumab on LDL-C was generally similar to that observed among adult patients with [[HoFH]]. | |||
* Including experience from open-label, uncontrolled studies, a total of 14 adolescents with [[HoFH]] have been treated with Evolocumab, with a median exposure duration of 9 months. | |||
* The safety profile of Evolocumab in these adolescents was similar to that described for adult patients with [[HoFH]]. | |||
* The safety and effectiveness of Evolocumab have not been established in pediatric patients with [[HoFH]] who are younger than 13 years old. | |||
* The safety and effectiveness of Evolocumab have not been established in pediatric patients with [[primary hyperlipidemia]] or [[HeFH]]. | |||
|useInGeri==== Geriatric Use === | |||
* In controlled studies, 1420 patients treated with Evolocumab were ≥ 65 years old and 171 were ≥ 75 years old. | |||
* No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. | |||
|useInRenalImpair==== Renal Impairment === | |||
* No dose adjustment is needed in patients with mild to moderate renal impairment. No data are available in patients with severe renal impairment. | |||
|useInHepaticImpair==== Hepatic Impairment === | |||
* No dose adjustment is needed in patients with mild to moderate hepatic impairment ([[Child-Pugh]] A or B). | |||
* No data are available in patients with severe hepatic impairment. | |||
<!--Administration and Monitoring--> | |||
|administration=* The 420 mg dose of Evolocumab can be administered: | |||
** over 9 minutes by using the single-use on-body infusor with prefilled cartridge, or | |||
** by giving 3 injections consecutively within 30 minutes using the single-use prefilled autoinjector or single-use prefilled syringe. | |||
* Provide proper training to patients and/or caregivers on how to prepare and administer Evolocumab prior to use, according to the Instructions for Use, including aseptic technique. | |||
* Instruct patients and/or caregivers to read and follow the Instructions for Use each time they use Evolocumab. | |||
* Keep Evolocumab in the refrigerator. | |||
* Prior to use, allow Evolocumab to warm to room temperature for at least 30 minutes for the single-use prefilled autoinjector or single-use prefilled syringe and for at least 45 minutes for the single-use on-body infusor with prefilled cartridge. | |||
* Do not warm in any other way. | |||
* Alternatively, for patients and caregivers, Evolocumab can be kept at room temperature at 68°F to 77°F (20°C to 25°C) in the original carton. | |||
* However, under these conditions, Evolocumab must be used within 30 days. | |||
* Visually inspect Evolocumab for particles and discoloration prior to administration. | |||
* Evolocumab is a clear to opalescent, colorless to pale yellow solution. | |||
* Do not use if the solution is cloudy or discolored or contains particles. | |||
* Administer Evolocumab subcutaneously into areas of the abdomen, thigh, or upper arm that are not tender, bruised, red, or indurated using a single-use prefilled syringe, single-use prefilled autoinjector, or single-use on-body infusor with prefilled cartridge. | |||
* Do not co-administer Evolocumab with other injectable drugs at the same administration site. | |||
* Rotate the site of each subcutaneous administration. | |||
|monitoring=* The recommended subcutaneous dosage of Evolocumab in patients with HeFH or patients with [[primary hyperlipidemia]] with established clinical [[atherosclerotic CVD]] is either 140 mg every 2 weeks OR 420 mg once monthly. | |||
* When switching dosage regimens, administer the first dose of the new regimen on the next scheduled date of the prior regimen. | |||
* The recommended subcutaneous dosage of Evolocumab in patients with [[HoFH]] is 420 mg once monthly. | |||
* In patients with [[HoFH]], measure [[LDL-C]] levels 4 to 8 weeks after starting REPATHA, since response to therapy will depend on the degree of [[LDL-receptor]] function. | |||
* If an every 2 week or once monthly dose is missed, instruct the patient to: | |||
** Administer Evolocumab as soon as possible if there are more than 7 days until the next scheduled dose, or, | |||
Omit the missed dose and administer the next dose according to the original schedule. | |||
<!--Drug box 2--> | |||
<!--Mechanism of Action--> | |||
|mechAction=* Evolocumab is a human monoclonal [[IgG2]] directed against human [[proprotein convertase subtilisin kexin 9]] ([[PCSK9]]). | |||
* Evolocumab binds to [[PCSK9]] and inhibits circulating PCSK9 from binding to the [[low density lipoprotein]] (LDL) receptor (LDLR), preventing PCSK9-mediated LDLR degradation and permitting LDLR to recycle back to the liver cell surface. | |||
* By inhibiting the binding of PCSK9 to LDLR, evolocumab increases the number of LDLRs available to clear [[LDL]] from the blood, thereby lowering LDL-C levels. | |||
<!--Structure--> | |||
|structure=* Evolocumab is a human monoclonal immunoglobulin G2 (IgG2) directed against human proprotein convertase subtilisin kexin 9 (PCSK9). | |||
* Evolocumab has an approximate molecular weight (MW) of 144 kDa and is produced in genetically engineered mammalian (Chinese hamster ovary) cells. | |||
* Evolocumab is a sterile, preservative-free, clear to opalescent, colorless to pale yellow solution for subcutaneous administration. | |||
* Each 1 mL single-use prefilled syringe and single-use prefilled SureClick® autoinjector contains 140 mg evolocumab, acetate (1.2 mg), polysorbate 80 (0.1 mg), proline (25 mg) in Water for Injection, USP. | |||
* Sodium hydroxide may be used to adjust to a pH of 5.0. | |||
* Each single-use PushtronexTM system (on-body infusor with prefilled cartridge) delivers a 3.5 mL solution containing 420 mg evolocumab, acetate (4.2 mg), polysorbate 80 (0.35 mg), proline (89 mg) in Water for Injection, USP. | |||
* Sodium hydroxide may be used to adjust to a pH of 5.0. | |||
<!--Pharmacodynamics--> | |||
|PD=* Following single subcutaneous administration of 140 mg or 420 mg of evolocumab, maximum suppression of circulating unbound PCSK9 occurred by 4 hours. | |||
* Unbound PCSK9 concentrations returned toward baseline when evolocumab concentrations decreased below the limit of quantitation. | |||
<!--Pharmacokinetics--> | |||
|PK=* Evolocumab exhibits non-linear kinetics as a result of binding to PCSK9. | |||
* Administration of the 140 mg dose in healthy volunteers resulted in a Cmax mean (standard deviation [SD]) of 18.6 (7.3) μg/mL and AUClast mean (SD) of 188 (98.6) day•μg/mL. | |||
* Administration of the 420 mg dose in healthy volunteers resulted in a Cmax mean (SD) of 59.0 (17.2) μg/mL and AUClast mean (SD) of 924 (346) day•μg/mL. | |||
* Following a single 420 mg intravenous dose, the mean (SD) systemic clearance was estimated to be 12 (2) mL/hr. | |||
* An approximate 2- to 3-fold accumulation was observed in trough serum concentrations (Cmin [SD] 7.21 [6.6]) following 140 mg doses administered subcutaneously every 2 weeks or following 420 mg doses administered subcutaneously monthly (Cmin [SD] 11.2 [10.8]), and serum trough concentrations approached steady state by 12 weeks of dosing. | |||
* '''Absorption''' | |||
:* Following a single subcutaneous dose of 140 mg or 420 mg evolocumab administered to healthy adults, median peak serum concentrations were attained in 3 to 4 days, and estimated absolute bioavailability was 72%. | |||
* '''Distribution''' | |||
:* Following a single 420 mg intravenous dose, the mean (SD) steady-state volume of distribution was estimated to be 3.3 (0.5) L. | |||
* Metabolism and Elimination | |||
:* Two elimination phases were observed for Evolocumab. | |||
:* At low concentrations, the elimination is predominately through saturable binding to target (PCSK9), while at higher concentrations the elimination of Evolocumab is largely through a non-saturable proteolytic pathway. | |||
:* Evolocumab was estimated to have an effective half-life of 11 to 17 days. | |||
* Specific Populations | |||
:* The pharmacokinetics of evolocumab were not affected by age, gender, race, or creatinine clearance, across all approved populations [see Use in Specific Populations (8.5)]. | |||
:* The exposure of evolocumab decreased with increasing body weight. | |||
:* These differences are not clinically meaningful. | |||
* '''Renal Impairment''' | |||
:* Since monoclonal antibodies are not known to be eliminated via renal pathways, renal function is not expected to impact the pharmacokinetics of evolocumab. | |||
:* Patients with severe renal impairment (estimated [[glomerular filtration rate]] [eGFR] < 30 mL/min/1.73 m2) have not been studied. | |||
* Hepatic Impairment | |||
:* Following a single 140 mg subcutaneous dose of evolocumab in patients with mild or moderate hepatic impairment, a 20-30% lower mean Cmax and 40-50% lower mean AUC were observed as compared to healthy patients; however, no dose adjustment is necessary in these patients. | |||
* Pregnancy | |||
:* The effect of pregnancy on evolocumab pharmacokinetics has not been studied. | |||
* Drug Interaction Studies | |||
:* An approximately 20% decrease in the Cmax and AUC of evolocumab was observed in patients co-administered with a high-intensity statin regimen. | |||
:* This difference is not clinically meaningful and does not impact dosing recommendations. | |||
<!--Nonclinical Toxicology--> | |||
|nonClinToxic==== Carcinogenesis, Mutagenesis, Impairment of Fertility === | |||
* The carcinogenic potential of evolocumab was evaluated in a lifetime study conducted in the hamster at dose levels of 10, 30, and 100 mg/kg administered every 2 weeks. | |||
* There were no evolocumab-related tumors at the highest dose at systemic exposures up to 38- and 15-fold the recommended human doses of 140 mg every 2 weeks and 420 mg once monthly, respectively, based on plasma AUC. | |||
* The mutagenic potential of evolocumab has not been evaluated; however, [[monoclonal antibodies]] are not expected to alter DNA or chromosomes. | |||
* There were no adverse effects on fertility (including estrous cycling, sperm analysis, mating performance, and embryonic development) at the highest dose in a fertility and early embryonic developmental toxicology study in hamsters when evolocumab was subcutaneously administered at 10, 30, and 100 mg/kg every 2 weeks. | |||
* The highest dose tested corresponds to systemic exposures up to 30- and 12-fold the recommended human doses of 140 mg every 2 weeks and 420 mg once monthly, respectively, based on plasma AUC. | |||
* In addition, there were no adverse evolocumab-related effects on surrogate markers of fertility (reproductive organ histopathology, menstrual cycling, or sperm parameters) in a 6-month chronic toxicology study in sexually mature monkeys subcutaneously administered evolocumab at 3, 30, and 300 mg/kg once weekly. | |||
* The highest dose tested corresponds to 744- and 300-fold the recommended human doses of 140 mg every 2 weeks and 420 mg once monthly, respectively, based on plasma AUC. | |||
==== Animal Toxicology and/or Pharmacology ==== | |||
* During a 3-month toxicology study of 10 and 100 mg/kg once every 2 weeks evolocumab in combination with 5 mg/kg once daily [[rosuvastatin]] in adult monkeys, there were no effects of evolocumab on the humoral immune response to keyhole limpet hemocyanin (KLH) after 1 to 2 months exposure. | |||
* The highest dose tested corresponds to exposures 54- and 21-fold higher than the recommended human doses of 140 mg every 2 weeks and 420 mg once monthly, respectively, based on plasma AUC. | |||
* Similarly, there were no effects of evolocumab on the [[humoral immune response]] to KLH (after 3 to 4 months exposure) in a 6-month study in cynomolgus monkeys at dose levels up to 300 mg/kg once weekly evolocumab corresponding to exposures 744- and 300-fold greater than the recommended human doses of 140 mg every 2 weeks and 420 mg once monthly, respectively, based on plasma AUC. | |||
<!--Clinical Studies--> | |||
|clinicalStudies==== Clinical Studies === | |||
==== Primary Hyperlipidemia in Patients with Clinical Atherosclerotic Cardiovascular Disease ==== | |||
* Study 1 was a multicenter, double-blind, randomized controlled trial in which patients were initially randomized to an open-label specific statin regimen for a 4-week lipid stabilization period followed by random assignment to subcutaneous injections of Evolocumab 140 mg every 2 weeks, Evolocumab 420 mg once monthly, or placebo for 12 weeks. | |||
* The trial included 296 patients with [[atherosclerotic CVD]] who received Evolocumab or placebo as add-on therapy to daily doses of [[atorvastatin]] 80 mg, [[rosuvastatin]] 40 mg, or [[simvastatin]] 40 mg. | |||
* Among these patients, the mean age at baseline was 63 years (range: 32 to 80 years), 45% were ≥ 65 years old, 33% women, 98% White, 2% were Black, < 1% Asian and 5% Hispanic or Latino. | |||
* After 4 weeks of statin therapy, the mean baseline LDL-C was 108 mg/dL. | |||
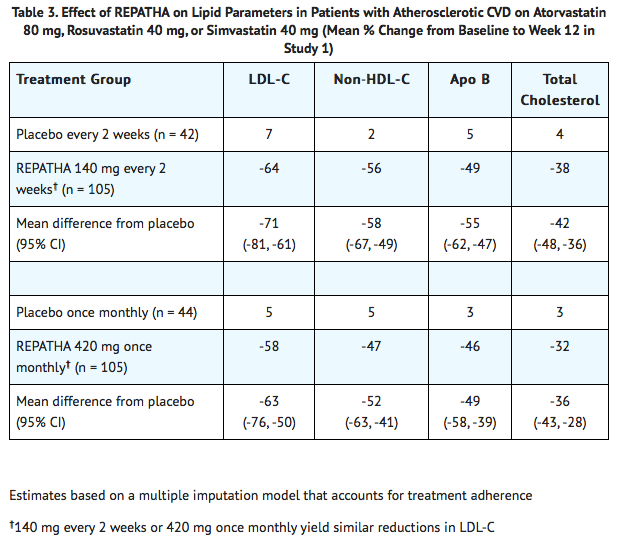
* In these patients with atherosclerotic CVD who were on maximum-dose statin therapy, the difference between Evolocumab and placebo in mean percent change in LDL-C from baseline to Week 12 was -71% (95% CI: -81%, -61%; p < 0.0001) and -63% (95% CI: -76%, -50%; p ˂ 0.0001) for the 140 mg every 2 weeks and 420 mg once monthly dosages, respectively. For additional results see Table 3 and Figure 1. | |||
[[File:Repatha CS.png|thumb|center|This image is provided by the National Library of Medicine]] | |||
[[File:REPATHA CS.png|thumb|center|This image is provided by the National Library of Medicine]] | |||
* Study 2 was a multicenter, double-blind, randomized, placebo-controlled, 52-week trial that included 139 patients with atherosclerotic CVD who received protocol-determined background lipid-lowering therapy of [[atorvastatin]] 80 mg daily with or without [[ezetimibe]] 10 mg daily. | |||
* After stabilization on background therapy, patients were randomly assigned to the addition of placebo or Evolocumab 420 mg administered subcutaneously once monthly. | |||
* Among these patients, the mean age at baseline was 59 years (range: 35 to 75 years), 25% were ≥ 65 years, 40% women, 80% White, 3% Black, 5% Asian, and < 1% Hispanic or Latino. | |||
* After stabilization on the assigned background therapy, the mean baseline LDL-C was 105 mg/dL. | |||
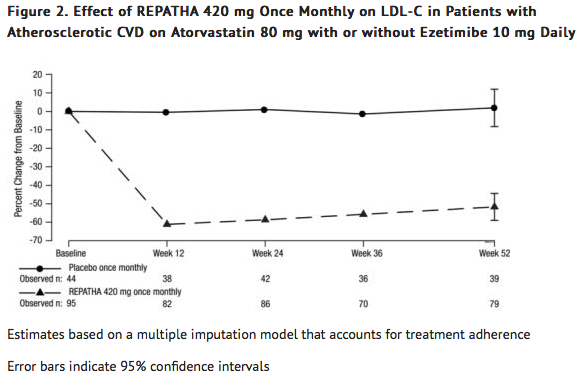
* In these patients with atherosclerotic CVD on maximum-dose atorvastatin therapy with or without [[ezetimibe]], the difference between Evolocumab 420 mg once monthly and placebo in mean percent change in LDL-C from baseline to Week 52 was -54 % (95% CI: -65%, -42%; p ˂ 0.0001) (Table 4 and Figure 2). | |||
* For additional results see Table 4. | |||
[[File:EVOLOCUMAB-CS.png|thumb|center|This image is provided by the National Library of Medicine]] | |||
[[File:EVOLOCUMAB.png|thumb|center|This image is provided by the National Library of Medicine]] | |||
==== Heterozygous Familial Hypercholesterolemia (HeFH) ==== | |||
* Study 3 was a multicenter, double-blind, randomized, placebo-controlled, 12-week trial in 329 patients with heterozygous [[familial hypercholesterolemia]] ([[HeFH]]) on statins with or without other lipid-lowering therapies. | |||
* Patients were randomized to receive subcutaneous injections of Evolocumab 140 mg every two weeks, 420 mg once monthly, or placebo. | |||
* [[HeFH]] was diagnosed by the Simon Broome criteria (1991). | |||
* In Study 3, 38% of patients had clinical atherosclerotic cardiovascular disease. | |||
* The mean age at baseline was 51 years (range: 19 to 79 years), 15% of the patients were ≥ 65 years old, 42% were women, 90% were White, 5% were Asian, and 1% were Black. | |||
* The average [[LDL-C]] at baseline was 156 mg/dL with 76% of the patients on high-intensity statin therapy. | |||
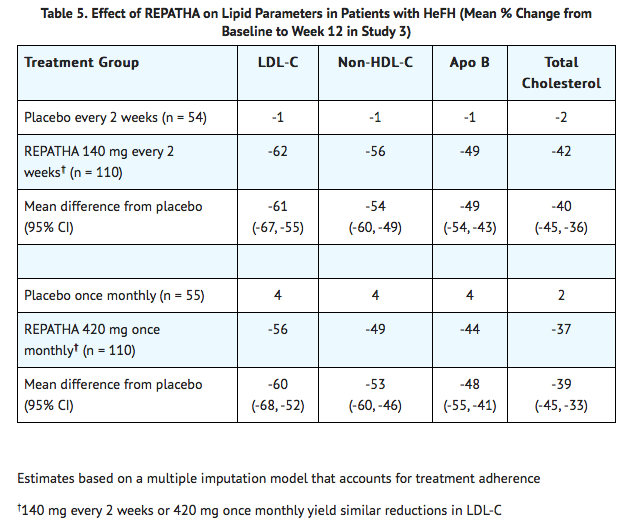
* In these patients with [[HeFH]] on statins with or without other lipid lowering therapies, the differences between REPATHA and placebo in mean percent change in LDL-C from baseline to Week 12 was -61% (95% CI: -67%, -55%; p < 0.0001) and -60% (95% CI: -68%, -52%; p < 0.0001) for the 140 mg every 2 weeks and 420 mg once monthly dosages, respectively. | |||
* For additional results see Table 5. | |||
[[File:EVOLOCUMAB-HeFH.png|thumb|center|This image is provided by the National Library of Medicine]] | |||
==== Homozygous Familial Hypercholesterolemia (HoFH) ==== | |||
* Study 4 was a multicenter, double-blind, randomized, placebo-controlled, 12-week trial in 49 patients (not on lipid-apheresis therapy) with homozygous [[familial hypercholesterolemia]] (HoFH). | |||
* In this trial, 33 patients received subcutaneous injections of 420 mg of Evolocumab once monthly and 16 patients received placebo as an adjunct to other lipid-lowering therapies (e.g., statins, [[ezetimibe]]). | |||
* The mean age at baseline was 31 years, 49% were women, 90% White, 4% were Asian, and 6% other. | |||
* The trial included 10 adolescents (ages 13 to 17 years), 7 of whom received Evolocumab. | |||
* The mean LDL-C at baseline was 349 mg/dL with all patients on statins ([[atorvastatin]] or [[rosuvastatin]]) and 92% on [[ezetimibe]]. | |||
* The diagnosis of [[HoFH]] was made by genetic confirmation or a clinical diagnosis based on a history of an untreated LDL-C concentration > 500 mg/dL together with either xanthoma before 10 years of age or evidence of [[HeFH]] in both parents. | |||
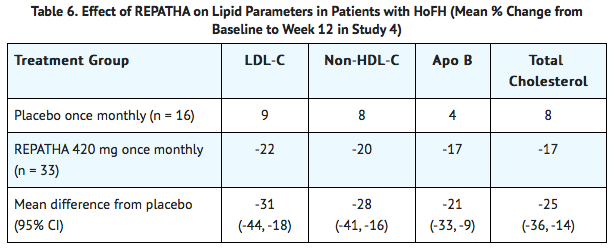
* In these patients with HoFH, the difference between Evolocumab and placebo in mean percent change in LDL-C from baseline to Week 12 was -31% (95% CI: -44%, -18%; p < 0.0001). | |||
* For additional results see Table 6. | |||
* Patients known to have two LDL-receptor negative alleles (little to no residual function) did not respond to Evolocumab. | |||
[[File:EVOLOCUMAB-HoFH.png|thumb|center|This image is provided by the National Library of Medicine]] | |||
<!--How Supplied--> | |||
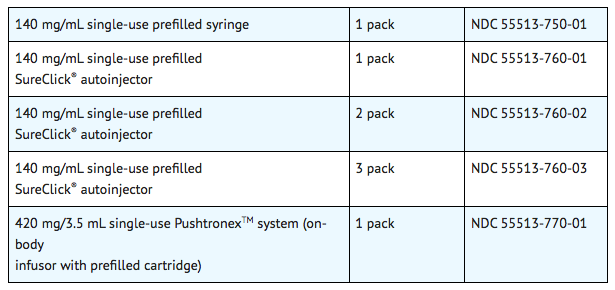
|howSupplied=* Evolocumab is a sterile, clear to opalescent, colorless to pale yellow solution for subcutaneous administration supplied in a single-use prefilled syringe, a single-use prefilled SureClick® autoinjector, or a single-use PushtronexTM system (on-body infusor with prefilled cartridge). | |||
* Each single-use prefilled syringe or single-use prefilled SureClick® autoinjector of Evolocumab is designed to deliver 1 mL of 140 mg/mL solution. | |||
* Each single-use PushtronexTM system (on-body infusor with prefilled cartridge) is designed to deliver 420 mg evolocumab in 3.5 mL solution. | |||
[[File:EVOLOCUMAB-Supply.png|thumb|center|This image is provided by the National Library of Medicine]] | |||
|storage* Pharmacy | |||
:* Store refrigerated at 2°C to 8°C (36° to 46°F) in the original carton to protect from light. | |||
:* Do not freeze. | |||
:* Do not shake. | |||
* For Patients/Caregivers | |||
:* Store refrigerated at 2°C to 8°C (36° to 46°F) in the original carton. | |||
:* Alternatively, Evolocumab can be kept at room temperature at 68°F to 77°F (20°C to 25°C) in the original carton; however, under these conditions, Evolocumab must be used within 30 days. | |||
:* If not used within the 30 days, discard Evolocumab. | |||
:* Protect Evolocumab from direct light and do not expose to temperatures above 25°C (77°F). | |||
<!--Label Display Image--> | |||
|packLabel=[[File:EVOLOCUMAB140.png|400px|thumb|center|This image is provided by the National Library of Medicine]] | |||
[[File:EVOLOCUMAB11.png|400px|thumb|center|This image is provided by the National Library of Medicine]] | |||
[[File:EVOLOCUMAB135.png|400px|thumb|center|This image is provided by the National Library of Medicine]] | |||
<!--Patient Counseling Information--> | |||
|fdaPatientInfo=* Advise the patient and/or caregiver to read the FDA-approved patient labeling [Patient Information and Instructions for Use (IFU)] before the patient starts using Evolocumab, and each time the patient gets a refill as there may be new information they need to know. | |||
* Provide guidance to patients and caregivers on proper subcutaneous administration technique, including aseptic technique, and how to use the single-use prefilled autoinjector, single-use prefilled syringe, or single-use on-body infusor with prefilled cartridge correctly (see Instructions for Use leaflet). | |||
* Inform patients that it may take up to 15 seconds to administer Evolocumab using the single-use prefilled autoinjector or single-use prefilled syringe and about 9 minutes to administer Evolocumab using the single-use on-body infusor with prefilled cartridge. | |||
* Advise latex-sensitive patients that the following components contain dry natural rubber (a derivative of latex) that may cause allergic reactions in individuals sensitive to latex: the needle cover of the glass single-use prefilled syringe and the single-use prefilled autoinjector. | |||
* The single-use on-body infusor with prefilled cartridge is not made with natural rubber latex. | |||
|brandNames=REPATHA® (evolocumab)}} | |||
Latest revision as of 23:27, 18 March 2017
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Shivani Chaparala M.B.B.S [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Evolocumab is a PCSK9 (proprotein convertase subtilisin kexin type9) inhibitor antibody that is FDA approved for the treatment of Primary Hyperlipidemia, Homozygous Familial Hypercholesterolemia, Heterozygous Familial Hypercholesterolemia, or Clinical atherosclerotic cardiovascular disease (CVD).. Common adverse reactions include Nasopharyngitis, upper respiratory tract infection, Influenza, back pain, and injection site reactions..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Primary Hyperlipidemia
- Evolocumab is indicated as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or clinical atherosclerotic cardiovascular disease (CVD), who require additional lowering of low density lipoprotein cholesterol (LDL-C).
Homozygous Familial Hypercholesterolemia
- Evolocumab is indicated as an adjunct to diet and other LDL-lowering therapies (e.g., statins, ezetimibe, LDL apheresis) for the treatment of patients with homozygous familial hypercholesterolemia (HoFH) who require additional lowering of LDL-C.
Dosage forms and strengths
- Evolocumab is a sterile, clear to opalescent, colorless to pale yellow solution available as follows:
- Injection: 140 mg/mL solution in a single-use profiled syringe.
- Injection: 140 mg/mL solution in a single-use prefilled SureClick® auto injector.
- Injection: 420 mg/3.5 mL solution in a single-use PushtronexTM system (on-body infusor with prefilled cartridge).
Off-Label Use and Dosage (Adult)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Evolocumab FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Contraindications
- Evolocumab is contraindicated in patients with a history of a serious hypersensitivity reaction to Evolocumab.
Warnings
Allergic Reactions
- Hypersensitivity reactions (e.g., rash, urticaria) have been reported in patients treated with Evolocumab, including some that led to discontinuation of therapy.
- If signs or symptoms of serious allergic reactions occur, discontinue treatment with Evolocumab, treat according to the standard of care, and monitor until signs and symptoms resolve.
Adverse Reactions
Clinical Trials Experience
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- Adverse Reactions in Patients with Primary Hyperlipidemia and in Patients with Heterozygous Familial Hypercholesterolemia
- Evolocumab is not indicated for use in patients without familial hypercholesterolemia or atherosclerotic CVD.
- The data described below reflect exposure to Evolocumab in 8 placebo-controlled trials that included 2651 patients treated with Evolocumab, including 557 exposed for 6 months and 515 exposed for 1 year (median treatment duration of 12 weeks).
- The mean age of the population was 57 years, 49% of the population were women, 85% White, 6% Black, 8% Asians, and 2% other races.
- Adverse Reactions in a 52-Week Controlled Trial
- In a 52-week, double-blind, randomized, placebo-controlled trial (Study 2), 599 patients received 420 mg of Evolocumab subcutaneously once monthly.
- The mean age was 56 years (range: 22 to 75 years), 23% were older than 65 years, 52% women, 80% White, 8% Black, 6% Asian, and 6% Hispanic.
- Adverse reactions reported in at least 3% of Evolocumab-treated patients, and more frequently than in placebo-treated patients in Study 2, are shown in Table 1.
- Adverse reactions led to discontinuation of treatment in 2.2% of Evolocumab-treated patients and 1% of placebo-treated patients.
- The most common adverse reaction that led to Evolocumab treatment discontinuation and occurred at a rate greater than placebo was myalgia (0.3% versus 0% for Evolocumab and placebo, respectively).

† includes erythema, pain, bruising.
- Adverse Reactions in Seven Pooled 12-Week Controlled Trials
- In seven pooled 12-week, double-blind, randomized, placebo-controlled trials, 993 patients received 140 mg of Evolocumab subcutaneously every 2 weeks and 1059 patients received 420 mg of Evolocumab subcutaneously monthly.
- The mean age was 57 years (range: 18 to 80 years), 29% were older than 65 years, 49% women, 85% White, 5% Black, 9% Asian, and 5% Hispanic.
- Adverse reactions reported in at least 1% of Evolocumab-treated patients, and more frequently than in placebo-treated patients, are shown in Table 2.

- Adverse Reactions in Eight Pooled Controlled Trials (Seven 12-Week Trials and One 52-Week Trial)
- The adverse reactions described below are from a pool of the 52-week trial (Study 2) and seven 12-week trials.
- The mean and median exposure durations of Evolocumab in this pool of eight trials were 20 weeks and 12 weeks, respectively.
- Local Injection Site Reactions
- Injection site reactions occurred in 3.2% and 3.0% of Evolocumab-treated and placebo-treated patients, respectively.
- The most common injection site reactions were erythema, pain, and bruising.
- The proportions of patients who discontinued treatment due to local injection site reactions in Evolocumab-treated patients and placebo-treated patients were 0.1% and 0%, respectively.
- Allergic Reactions
- Allergic reactions occurred in 5.1% and 4.7% of Evolocumab-treated and placebo-treated patients, respectively.
- The most common allergic reactions were rash (1.0% versus 0.5% for Evolocumab and placebo, respectively), eczema (0.4% versus 0.2%), erythema (0.4% versus 0.2%), and urticaria (0.4% versus 0.1%).
- Neurocognitive Events
- In placebo-controlled trials, neurocognitive events were reported in less than or equal to 0.2% in Evolocumab-treated and placebo-treated patients.
- Low LDL-C Levels
- In a pool of placebo- and active-controlled trials, as well as open-label extension studies that followed them, a total of 1988 patients treated with Evolocumab had at least one LDL-C value < 25 mg/dL.
- Changes to background lipid-altering therapy were not made in response to low LDL-C values, and Evolocumab dosing was not modified or interrupted on this basis.
- Although adverse consequences of very low LDL-C were not identified in these trials, the long-term effects of very low levels of LDL-C induced by Evolocumab are unknown.
- Musculoskeletal Events
- Musculoskeletal adverse reactions were reported in 14.3% of Evolocumab-treated patients and 12.8% of placebo-treated patients.
- The most common adverse reactions that occurred at a rate greater than placebo were back pain (3.2% versus 2.9% for Evolocumab and placebo, respectively), arthralgia (2.3% versus 2.2%), and myalgia (2.0% versus 1.8%).
- Adverse Reactions in Patients with Homozygous Familial Hypercholesterolemia
- In a 12-week, double-blind, randomized, placebo-controlled trial of 49 patients with HoFH (Study 4), 33 patients received 420 mg of Evolocumab subcutaneously once monthly [see Clinical Studies (14.3)].
- The mean age was 31 years (range: 13 to 57 years), 49% were women, 90% White, 4% Asian, and 6% other.
- The adverse reactions that occurred in at least two (6.1%) Evolocumab-treated patients, and more frequently than in placebo-treated patients, included:
- Upper respiratory tract infection(9.1% versus 6.3%).
- Influenza (9.1% versus 0%).
- Gastroenteritis (6.1% versus 0%).
- Nasopharyngitis (6.1% versus 0%).
Immunogenicity
- As with all therapeutic proteins, there is potential for immunogenicity.
- The immunogenicity of Evolocumab has been evaluated using an electrochemiluminescent bridging screening immunoassay for the detection of binding anti-drug antibodies.
- For patients whose sera tested positive in the screening immunoassay, an in vitro biological assay was performed to detect neutralizing antibodies.
- In a pool of placebo- and active-controlled clinical trials, 0.1% of patients treated with at least one dose of Evolocumab tested positive for binding antibody development.
- Patients whose sera tested positive for binding antibodies were further evaluated for neutralizing antibodies; none of the patients tested positive for neutralizing antibodies.
- There was no evidence that the presence of anti-drug binding antibodies impacted the pharmacokinetic profile, clinical response, or safety of Evolocumab, but the long-term consequences of continuing Evolocumab treatment in the presence of anti-drug binding antibodies are unknown.
- The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay.
- Additionally, the observed incidence of antibody positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease.
- For these reasons, comparison of the incidence of antibodies to Evolocumab with the incidence of antibodies to other products may be misleading.
Postmarketing Experience
There is limited information regarding Evolocumab Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Evolocumab Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy
- Risk Summary
- There are no data available on use of Evolocumab in pregnant women to inform a drug-associated risk.
- In animal reproduction studies, there were no effects on pregnancy or neonatal/infant development when monkeys were subcutaneously administered evolocumab from organogenesis through parturition at dose exposures up to 12 times the exposure at the maximum recommended human dose of 420 mg every month.
- In a similar study with another drug in the PCSK9 inhibitor antibody class, humoral immune suppression was observed in infant monkeys exposed to that drug in utero at all doses.
- The exposures where immune suppression occurred in infant monkeys were greater than those expected clinically.
- No assessment for immune suppression was conducted with evolocumab in infant monkeys.
- Measurable evolocumab serum concentrations were observed in the infant monkeys at birth at comparable levels to maternal serum, indicating that evolocumab, like other IgG antibodies, crosses the placental barrier.
- FDA’s experience with monoclonal antibodies in humans indicates that they are unlikely to cross the placenta in the first trimester; however, they are likely to cross the placenta in increasing amounts in the second and third trimester.
- Consider the benefits and risks of Evolocumab and possible risks to the fetus before prescribing Evolocumab to pregnant women.
- In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
- Data
- Animal Data
- In cynomolgus monkeys, no effects on embryo-fetal or postnatal development (up to 6 months of age) were observed when evolocumab was dosed during organogenesis to parturition at 50 mg/kg once every 2 weeks by the subcutaneous route at exposures 30- and 12-fold the recommended human doses of 140 mg every 2 weeks and 420 mg once monthly, respectively, based on plasma AUC.
- No test of humoral immunity in infant monkeys was conducted with evolocumab.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Evolocumab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Evolocumab during labor and delivery.
Nursing Mothers
Lactation
- Risk Summary
- There is no information regarding the presence of evolocumab in human milk, the effects on the breastfed infant, or the effects on milk production.
- The development and health benefits of breastfeeding should be considered along with the mother’s clinical need for Evolocumab and any potential adverse effects on the breastfed infant from Evolocumab or from the underlying maternal condition.
- Human IgG is present in human milk, but published data suggest that breast milk antibodies do not enter the neonatal and infant circulation in substantial amounts.
Pediatric Use
Pediatric Use
- The safety and effectiveness of Evolocumab in combination with diet and other LDL-C-lowering therapies in adolescents with HoFH who require additional lowering of LDL-C were established based on data from a 12-week, placebo-controlled trial that included 10 adolescents (ages 13 to 17 years old) with HoFH.
- In this trial, 7 adolescents received Evolocumab 420 mg subcutaneously once monthly and 3 adolescents received placebo.
- The effect of Evolocumab on LDL-C was generally similar to that observed among adult patients with HoFH.
- Including experience from open-label, uncontrolled studies, a total of 14 adolescents with HoFH have been treated with Evolocumab, with a median exposure duration of 9 months.
- The safety profile of Evolocumab in these adolescents was similar to that described for adult patients with HoFH.
- The safety and effectiveness of Evolocumab have not been established in pediatric patients with HoFH who are younger than 13 years old.
- The safety and effectiveness of Evolocumab have not been established in pediatric patients with primary hyperlipidemia or HeFH.
Geriatic Use
Geriatric Use
- In controlled studies, 1420 patients treated with Evolocumab were ≥ 65 years old and 171 were ≥ 75 years old.
- No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Gender
There is no FDA guidance on the use of Evolocumab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Evolocumab with respect to specific racial populations.
Renal Impairment
Renal Impairment
- No dose adjustment is needed in patients with mild to moderate renal impairment. No data are available in patients with severe renal impairment.
Hepatic Impairment
Hepatic Impairment
- No dose adjustment is needed in patients with mild to moderate hepatic impairment (Child-Pugh A or B).
- No data are available in patients with severe hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Evolocumab in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Evolocumab in patients who are immunocompromised.
Administration and Monitoring
Administration
- The 420 mg dose of Evolocumab can be administered:
** over 9 minutes by using the single-use on-body infusor with prefilled cartridge, or ** by giving 3 injections consecutively within 30 minutes using the single-use prefilled autoinjector or single-use prefilled syringe.
- Provide proper training to patients and/or caregivers on how to prepare and administer Evolocumab prior to use, according to the Instructions for Use, including aseptic technique.
- Instruct patients and/or caregivers to read and follow the Instructions for Use each time they use Evolocumab.
- Keep Evolocumab in the refrigerator.
- Prior to use, allow Evolocumab to warm to room temperature for at least 30 minutes for the single-use prefilled autoinjector or single-use prefilled syringe and for at least 45 minutes for the single-use on-body infusor with prefilled cartridge.
- Do not warm in any other way.
- Alternatively, for patients and caregivers, Evolocumab can be kept at room temperature at 68°F to 77°F (20°C to 25°C) in the original carton.
- However, under these conditions, Evolocumab must be used within 30 days.
- Visually inspect Evolocumab for particles and discoloration prior to administration.
- Evolocumab is a clear to opalescent, colorless to pale yellow solution.
- Do not use if the solution is cloudy or discolored or contains particles.
- Administer Evolocumab subcutaneously into areas of the abdomen, thigh, or upper arm that are not tender, bruised, red, or indurated using a single-use prefilled syringe, single-use prefilled autoinjector, or single-use on-body infusor with prefilled cartridge.
- Do not co-administer Evolocumab with other injectable drugs at the same administration site.
- Rotate the site of each subcutaneous administration.
Monitoring
- The recommended subcutaneous dosage of Evolocumab in patients with HeFH or patients with primary hyperlipidemia with established clinical atherosclerotic CVD is either 140 mg every 2 weeks OR 420 mg once monthly.
- When switching dosage regimens, administer the first dose of the new regimen on the next scheduled date of the prior regimen.
- The recommended subcutaneous dosage of Evolocumab in patients with HoFH is 420 mg once monthly.
- In patients with HoFH, measure LDL-C levels 4 to 8 weeks after starting REPATHA, since response to therapy will depend on the degree of LDL-receptor function.
- If an every 2 week or once monthly dose is missed, instruct the patient to:
- Administer Evolocumab as soon as possible if there are more than 7 days until the next scheduled dose, or,
Omit the missed dose and administer the next dose according to the original schedule.
IV Compatibility
There is limited information regarding the compatibility of Evolocumab and IV administrations.
Overdosage
There is limited information regarding Evolocumab overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Evolocumab Pharmacology in the drug label.
Mechanism of Action
- Evolocumab is a human monoclonal IgG2 directed against human proprotein convertase subtilisin kexin 9 (PCSK9).
- Evolocumab binds to PCSK9 and inhibits circulating PCSK9 from binding to the low density lipoprotein (LDL) receptor (LDLR), preventing PCSK9-mediated LDLR degradation and permitting LDLR to recycle back to the liver cell surface.
- By inhibiting the binding of PCSK9 to LDLR, evolocumab increases the number of LDLRs available to clear LDL from the blood, thereby lowering LDL-C levels.
Structure
- Evolocumab is a human monoclonal immunoglobulin G2 (IgG2) directed against human proprotein convertase subtilisin kexin 9 (PCSK9).
- Evolocumab has an approximate molecular weight (MW) of 144 kDa and is produced in genetically engineered mammalian (Chinese hamster ovary) cells.
- Evolocumab is a sterile, preservative-free, clear to opalescent, colorless to pale yellow solution for subcutaneous administration.
- Each 1 mL single-use prefilled syringe and single-use prefilled SureClick® autoinjector contains 140 mg evolocumab, acetate (1.2 mg), polysorbate 80 (0.1 mg), proline (25 mg) in Water for Injection, USP.
- Sodium hydroxide may be used to adjust to a pH of 5.0.
- Each single-use PushtronexTM system (on-body infusor with prefilled cartridge) delivers a 3.5 mL solution containing 420 mg evolocumab, acetate (4.2 mg), polysorbate 80 (0.35 mg), proline (89 mg) in Water for Injection, USP.
- Sodium hydroxide may be used to adjust to a pH of 5.0.
Pharmacodynamics
- Following single subcutaneous administration of 140 mg or 420 mg of evolocumab, maximum suppression of circulating unbound PCSK9 occurred by 4 hours.
- Unbound PCSK9 concentrations returned toward baseline when evolocumab concentrations decreased below the limit of quantitation.
Pharmacokinetics
- Evolocumab exhibits non-linear kinetics as a result of binding to PCSK9.
- Administration of the 140 mg dose in healthy volunteers resulted in a Cmax mean (standard deviation [SD]) of 18.6 (7.3) μg/mL and AUClast mean (SD) of 188 (98.6) day•μg/mL.
- Administration of the 420 mg dose in healthy volunteers resulted in a Cmax mean (SD) of 59.0 (17.2) μg/mL and AUClast mean (SD) of 924 (346) day•μg/mL.
- Following a single 420 mg intravenous dose, the mean (SD) systemic clearance was estimated to be 12 (2) mL/hr.
- An approximate 2- to 3-fold accumulation was observed in trough serum concentrations (Cmin [SD] 7.21 [6.6]) following 140 mg doses administered subcutaneously every 2 weeks or following 420 mg doses administered subcutaneously monthly (Cmin [SD] 11.2 [10.8]), and serum trough concentrations approached steady state by 12 weeks of dosing.
- Absorption
- Following a single subcutaneous dose of 140 mg or 420 mg evolocumab administered to healthy adults, median peak serum concentrations were attained in 3 to 4 days, and estimated absolute bioavailability was 72%.
- Distribution
- Following a single 420 mg intravenous dose, the mean (SD) steady-state volume of distribution was estimated to be 3.3 (0.5) L.
- Metabolism and Elimination
- Two elimination phases were observed for Evolocumab.
- At low concentrations, the elimination is predominately through saturable binding to target (PCSK9), while at higher concentrations the elimination of Evolocumab is largely through a non-saturable proteolytic pathway.
- Evolocumab was estimated to have an effective half-life of 11 to 17 days.
- Specific Populations
- The pharmacokinetics of evolocumab were not affected by age, gender, race, or creatinine clearance, across all approved populations [see Use in Specific Populations (8.5)].
- The exposure of evolocumab decreased with increasing body weight.
- These differences are not clinically meaningful.
- Renal Impairment
- Since monoclonal antibodies are not known to be eliminated via renal pathways, renal function is not expected to impact the pharmacokinetics of evolocumab.
- Patients with severe renal impairment (estimated glomerular filtration rate [eGFR] < 30 mL/min/1.73 m2) have not been studied.
- Hepatic Impairment
- Following a single 140 mg subcutaneous dose of evolocumab in patients with mild or moderate hepatic impairment, a 20-30% lower mean Cmax and 40-50% lower mean AUC were observed as compared to healthy patients; however, no dose adjustment is necessary in these patients.
- Pregnancy
- The effect of pregnancy on evolocumab pharmacokinetics has not been studied.
- Drug Interaction Studies
- An approximately 20% decrease in the Cmax and AUC of evolocumab was observed in patients co-administered with a high-intensity statin regimen.
- This difference is not clinically meaningful and does not impact dosing recommendations.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- The carcinogenic potential of evolocumab was evaluated in a lifetime study conducted in the hamster at dose levels of 10, 30, and 100 mg/kg administered every 2 weeks.
- There were no evolocumab-related tumors at the highest dose at systemic exposures up to 38- and 15-fold the recommended human doses of 140 mg every 2 weeks and 420 mg once monthly, respectively, based on plasma AUC.
- The mutagenic potential of evolocumab has not been evaluated; however, monoclonal antibodies are not expected to alter DNA or chromosomes.
- There were no adverse effects on fertility (including estrous cycling, sperm analysis, mating performance, and embryonic development) at the highest dose in a fertility and early embryonic developmental toxicology study in hamsters when evolocumab was subcutaneously administered at 10, 30, and 100 mg/kg every 2 weeks.
- The highest dose tested corresponds to systemic exposures up to 30- and 12-fold the recommended human doses of 140 mg every 2 weeks and 420 mg once monthly, respectively, based on plasma AUC.
- In addition, there were no adverse evolocumab-related effects on surrogate markers of fertility (reproductive organ histopathology, menstrual cycling, or sperm parameters) in a 6-month chronic toxicology study in sexually mature monkeys subcutaneously administered evolocumab at 3, 30, and 300 mg/kg once weekly.
- The highest dose tested corresponds to 744- and 300-fold the recommended human doses of 140 mg every 2 weeks and 420 mg once monthly, respectively, based on plasma AUC.
Animal Toxicology and/or Pharmacology
- During a 3-month toxicology study of 10 and 100 mg/kg once every 2 weeks evolocumab in combination with 5 mg/kg once daily rosuvastatin in adult monkeys, there were no effects of evolocumab on the humoral immune response to keyhole limpet hemocyanin (KLH) after 1 to 2 months exposure.
- The highest dose tested corresponds to exposures 54- and 21-fold higher than the recommended human doses of 140 mg every 2 weeks and 420 mg once monthly, respectively, based on plasma AUC.
- Similarly, there were no effects of evolocumab on the humoral immune response to KLH (after 3 to 4 months exposure) in a 6-month study in cynomolgus monkeys at dose levels up to 300 mg/kg once weekly evolocumab corresponding to exposures 744- and 300-fold greater than the recommended human doses of 140 mg every 2 weeks and 420 mg once monthly, respectively, based on plasma AUC.
Clinical Studies
Clinical Studies
Primary Hyperlipidemia in Patients with Clinical Atherosclerotic Cardiovascular Disease
- Study 1 was a multicenter, double-blind, randomized controlled trial in which patients were initially randomized to an open-label specific statin regimen for a 4-week lipid stabilization period followed by random assignment to subcutaneous injections of Evolocumab 140 mg every 2 weeks, Evolocumab 420 mg once monthly, or placebo for 12 weeks.
- The trial included 296 patients with atherosclerotic CVD who received Evolocumab or placebo as add-on therapy to daily doses of atorvastatin 80 mg, rosuvastatin 40 mg, or simvastatin 40 mg.
- Among these patients, the mean age at baseline was 63 years (range: 32 to 80 years), 45% were ≥ 65 years old, 33% women, 98% White, 2% were Black, < 1% Asian and 5% Hispanic or Latino.
- After 4 weeks of statin therapy, the mean baseline LDL-C was 108 mg/dL.
- In these patients with atherosclerotic CVD who were on maximum-dose statin therapy, the difference between Evolocumab and placebo in mean percent change in LDL-C from baseline to Week 12 was -71% (95% CI: -81%, -61%; p < 0.0001) and -63% (95% CI: -76%, -50%; p ˂ 0.0001) for the 140 mg every 2 weeks and 420 mg once monthly dosages, respectively. For additional results see Table 3 and Figure 1.

- Study 2 was a multicenter, double-blind, randomized, placebo-controlled, 52-week trial that included 139 patients with atherosclerotic CVD who received protocol-determined background lipid-lowering therapy of atorvastatin 80 mg daily with or without ezetimibe 10 mg daily.
- After stabilization on background therapy, patients were randomly assigned to the addition of placebo or Evolocumab 420 mg administered subcutaneously once monthly.
- Among these patients, the mean age at baseline was 59 years (range: 35 to 75 years), 25% were ≥ 65 years, 40% women, 80% White, 3% Black, 5% Asian, and < 1% Hispanic or Latino.
- After stabilization on the assigned background therapy, the mean baseline LDL-C was 105 mg/dL.
- In these patients with atherosclerotic CVD on maximum-dose atorvastatin therapy with or without ezetimibe, the difference between Evolocumab 420 mg once monthly and placebo in mean percent change in LDL-C from baseline to Week 52 was -54 % (95% CI: -65%, -42%; p ˂ 0.0001) (Table 4 and Figure 2).
- For additional results see Table 4.

Heterozygous Familial Hypercholesterolemia (HeFH)
- Study 3 was a multicenter, double-blind, randomized, placebo-controlled, 12-week trial in 329 patients with heterozygous familial hypercholesterolemia (HeFH) on statins with or without other lipid-lowering therapies.
- Patients were randomized to receive subcutaneous injections of Evolocumab 140 mg every two weeks, 420 mg once monthly, or placebo.
- HeFH was diagnosed by the Simon Broome criteria (1991).
- In Study 3, 38% of patients had clinical atherosclerotic cardiovascular disease.
- The mean age at baseline was 51 years (range: 19 to 79 years), 15% of the patients were ≥ 65 years old, 42% were women, 90% were White, 5% were Asian, and 1% were Black.
- The average LDL-C at baseline was 156 mg/dL with 76% of the patients on high-intensity statin therapy.
- In these patients with HeFH on statins with or without other lipid lowering therapies, the differences between REPATHA and placebo in mean percent change in LDL-C from baseline to Week 12 was -61% (95% CI: -67%, -55%; p < 0.0001) and -60% (95% CI: -68%, -52%; p < 0.0001) for the 140 mg every 2 weeks and 420 mg once monthly dosages, respectively.
- For additional results see Table 5.
Homozygous Familial Hypercholesterolemia (HoFH)
- Study 4 was a multicenter, double-blind, randomized, placebo-controlled, 12-week trial in 49 patients (not on lipid-apheresis therapy) with homozygous familial hypercholesterolemia (HoFH).
- In this trial, 33 patients received subcutaneous injections of 420 mg of Evolocumab once monthly and 16 patients received placebo as an adjunct to other lipid-lowering therapies (e.g., statins, ezetimibe).
- The mean age at baseline was 31 years, 49% were women, 90% White, 4% were Asian, and 6% other.
- The trial included 10 adolescents (ages 13 to 17 years), 7 of whom received Evolocumab.
- The mean LDL-C at baseline was 349 mg/dL with all patients on statins (atorvastatin or rosuvastatin) and 92% on ezetimibe.
- The diagnosis of HoFH was made by genetic confirmation or a clinical diagnosis based on a history of an untreated LDL-C concentration > 500 mg/dL together with either xanthoma before 10 years of age or evidence of HeFH in both parents.
- In these patients with HoFH, the difference between Evolocumab and placebo in mean percent change in LDL-C from baseline to Week 12 was -31% (95% CI: -44%, -18%; p < 0.0001).
- For additional results see Table 6.
- Patients known to have two LDL-receptor negative alleles (little to no residual function) did not respond to Evolocumab.
How Supplied
- Evolocumab is a sterile, clear to opalescent, colorless to pale yellow solution for subcutaneous administration supplied in a single-use prefilled syringe, a single-use prefilled SureClick® autoinjector, or a single-use PushtronexTM system (on-body infusor with prefilled cartridge).
- Each single-use prefilled syringe or single-use prefilled SureClick® autoinjector of Evolocumab is designed to deliver 1 mL of 140 mg/mL solution.
- Each single-use PushtronexTM system (on-body infusor with prefilled cartridge) is designed to deliver 420 mg evolocumab in 3.5 mL solution.
Storage
There is limited information regarding Evolocumab Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Evolocumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Evolocumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient and/or caregiver to read the FDA-approved patient labeling [Patient Information and Instructions for Use (IFU)] before the patient starts using Evolocumab, and each time the patient gets a refill as there may be new information they need to know.
- Provide guidance to patients and caregivers on proper subcutaneous administration technique, including aseptic technique, and how to use the single-use prefilled autoinjector, single-use prefilled syringe, or single-use on-body infusor with prefilled cartridge correctly (see Instructions for Use leaflet).
- Inform patients that it may take up to 15 seconds to administer Evolocumab using the single-use prefilled autoinjector or single-use prefilled syringe and about 9 minutes to administer Evolocumab using the single-use on-body infusor with prefilled cartridge.
- Advise latex-sensitive patients that the following components contain dry natural rubber (a derivative of latex) that may cause allergic reactions in individuals sensitive to latex: the needle cover of the glass single-use prefilled syringe and the single-use prefilled autoinjector.
- The single-use on-body infusor with prefilled cartridge is not made with natural rubber latex.
Precautions with Alcohol
Alcohol-Evolocumab interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
REPATHA® (evolocumab)
Look-Alike Drug Names
There is limited information regarding Evolocumab Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.