Esmolol: Difference between revisions
No edit summary |
No edit summary |
||
| Line 190: | Line 190: | ||
Patients using [[beta blockers]] may be unresponsive to the usual doses of epinephrine used to treat [[anaphylactic]] or [[anaphylactoid]] reactions. | Patients using [[beta blockers]] may be unresponsive to the usual doses of epinephrine used to treat [[anaphylactic]] or [[anaphylactoid]] reactions. | ||
|clinicalTrials====== | |clinicalTrials======Clinical Trials Experience===== | ||
* Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. | |||
* The following adverse reaction rates are based on use of esmolol in clinical trials involving 369 patients with [[supraventricular tachycardia]] and over 600 intraoperative and postoperative patients enrolled in clinical trials. Most adverse effects observed in controlled clinical trial settings have been mild and transient. The most important and common adverse effect has been [[hypotension]]. Deaths have been reported in post-marketing experience occurring during complex clinical states where esmolol was presumably being used simply to control ventricular rate | |||
[[File:Esmololclinicaltrialsadverse.JPG|800px|thumbnail|left|This image is provided by the National Library of Medicine.]] | |||
{{clr}} | |||
=====Clinical Trial Adverse Reactions (Frequency <3%)===== | |||
======Psychiatric Disorders====== | |||
* [[Confusion]]al state and [[agitation]] (~2%) | |||
* [[Anxiety]], [[depression]] and abnormal thinking (<1%) | |||
====== | ======Nervous System Disorders====== | ||
* [[Headache]] (~ 2%) | |||
* [[Paresthesia]], [[syncope]], [[speech disorder]], and [[lightheadedness ]] (<1%) | |||
* Convulsions (<1%), with one death | |||
======Vascular Disorders====== | |||
* [[Peripheral ischemia]] (~1%) | |||
* [[Pallor]] and [[flushing]] (<1%) | |||
======Gastrointestinal====== | ======Gastrointestinal Disorders====== | ||
* [[Vomiting]] (~1%) | |||
* [[Dyspepsia]], [[constipation]], [[dry mouth]], and [[abdominal discomfort]] (<1%) | |||
======Renal and Urinary Disorders====== | |||
* [[Urinary retention]] (<1%) | |||
|postmarketing=In addition to the adverse reactions reported in clinical trials, the following adverse reactions have been reported in the post-marketing experience. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate reliably their frequency or to establish a causal relationship to drug exposure. | |||
====== | ===Cardiac Disorders=== | ||
* [[Cardiac arrest]], Coronary arteriospasm | |||
===Skin and Subcutaneous Tissue Disorders=== | |||
* [[Angioedema]], [[Urticaria]], [[Psoriasis]] | |||
|drugInteractions=* Drug 1 | |drugInteractions=* Drug 1 | ||
* Drug 2 | * Drug 2 | ||
Revision as of 15:21, 2 July 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]; Abdurahman Khalil, M.D. [3]; Alonso Alvarado, M.D. [4]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Esmolol is a beta-adrenergic blocker that is FDA approved for the {{{indicationType}}} of supraventricular tachycardiaor noncompensatory sinus tachycardia, intraoperative and postoperative tachycardia and/or hypertension. Common adverse reactions include hypotension, injection site pain, nausea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Supraventricular Tachycardia or Noncompensatory Sinus Tachycardia
- Dosing Information
- Esmolol is administered by continuous intravenous infusion with or without a loading dose. Additional loading doses and/or titration of the maintenance infusion (step-wise dosing) may be necessary based on desired ventricular response.
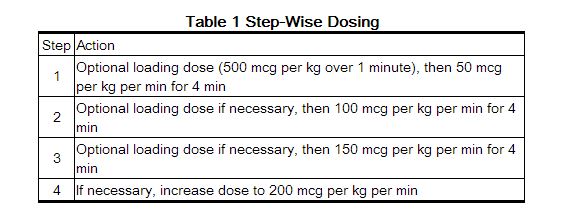
- In the absence of loading doses, continuous infusion of a single concentration of esmolol reaches pharmacokinetic and pharmacodynamic steady-state in about 30 minutes.
- The effective maintenance dose for continuous and step-wise dosing is 50 to 200 mcg per kg per minute, although doses as low as 25 mcg per kg per minute have been adequate. Dosages greater than 200 mcg per kg per minute provide little added heart rate lowering effect, and the rate of adverse reactions increases.
- Maintenance infusions may be continued for up to 48 hours.
Intraoperative and Postoperative Tachycardia and Hypertension
- Dosing Information
- In this setting it is not always advisable to slowly titrate to a therapeutic effect. Therefore two dosing options are presented: immediate control and gradual control.
=Immediate Control
- Administer 1 mg per kg as a bolus dose over 30 seconds followed by an infusion of 150 mcg per kg per min if necessary.
- Adjust the infusion rate as required to maintain desired heart rate and blood pressure. Refer to Maximum Recommended Doses below.
Gradual Control
- Administer 500 mcg per kg as a bolus dose over 1 minute followed by a maintenance infusion of 50 mcg per kg per min for 4 minutes.
- Depending on the response obtained, continue dosing as outlined for supraventricular tachycardia. Refer to Maximum Recommended Doses below.
Maximum Recommended Doses
- For the treatment of tachycardia, maintenance infusion dosages greater than 200 mcg per kg per min are not recommended; dosages greater than 200 mcg per kg per min provide little additional heart rate-lowering effect, and the rate of adverse reactions increases.
- For the treatment of hypertension, higher maintenance infusion dosages (250-300 mcg per kg per min) may be required. The safety of doses above 300 mcg per kg per minute has not been studied.
Transition from esmolol Injection Therapy to Alternative Drugs
- After patients achieve adequate control of the heart rate and a stable clinical status, transition to alternative antiarrhythmic drugs may be accomplished.
- When transitioning from BREVIBLOC to alternative drugs, the physician should carefully consider the labeling instructions of the alternative drug selected and reduce the dosage of esmolol as follows:
- Thirty minutes following the first dose of the alternative drug, reduce the esmolol infusion rate by one-half (50%).
- After administration of the second dose of the alternative drug, monitor the patient’s response and if satisfactory control is maintained for the first hour, discontinue the esmolol infusion.
Directions for Use
- Esmolol injection is available in a pre-mixed bag and ready-to-use vial. BREVIBLOC is not compatible with Sodium Bicarbonate (5%) solution (limited stability) or furosemide (precipitation).
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
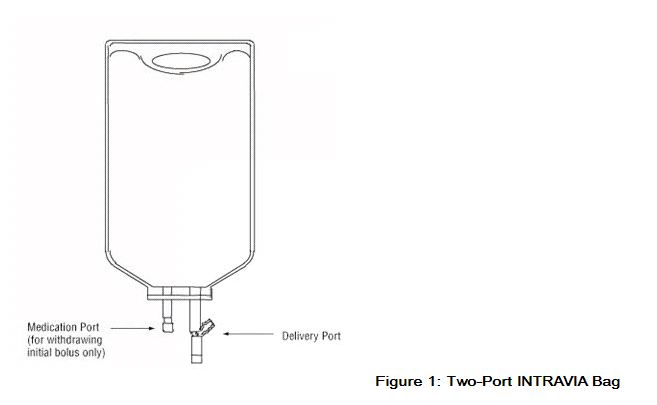
Premixed Bag
- The medication port is to be used solely for withdrawing an initial bolus from the bag.
- Use aseptic technique when withdrawing the bolus dose.
- Do not add any additional medications to the bag.
Ready-to-Use Vial
- The Ready-to-use Vial may be used to administer a loading dosage by hand-held syringe while the maintenance infusion is being prepared.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Esmolol FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Esmolol in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Esmolol in pediatric patients.
Contraindications
Esmolol is contraindicated in patients with:
- Severe sinus bradycardia: May precipitate or worsen bradycardia resulting in cardiogenic shock and cardiac arrest.
- Heart block greater than first degree: Second- or third-degree atrioventricular block may precipitate or worsen bradycardia resulting in cardiogenic shock and cardiac arrest.
- Sick sinus syndrome: May precipitate or worsen bradycardia resulting in cardiogenic shock and cardiac arrest.
- Decompensated heart failure: May worsen heart failure.
- Cardiogenic shock: May precipitate further cardiovascular collapse and cause cardiac arrest.
- IV administration of cardiodepressant calcium channel blockers (e.g., verapamil) and esmolol in close proximity (i.e., while cardiac effects from the other are still present); fatal cardiac arrests have occurred in patients receiving esmolol and intravenous verapamil.
- Pulmonary hypertension: May precipitate cardiorespiratory compromise.
- Hypersensitivity reactions, including anaphylaxis to esmolol or any of the inactive ingredients of the product (cross-sensitivity between beta blockers is possible).
Warnings
Hypotension
Hypotension can occur at any dose but is dose-related. Patients with hemodynamic compromise or on interacting medications are at particular risk. Severe reactions may include loss of consciousness, cardiac arrest, and death. For control of ventricular heart rate, maintenance doses greater than 200 mcg per kg per min are not recommended. Monitor patients closely, especially if pretreatment blood pressure is low. In case of an unacceptable drop in blood pressure, reduce or stop esmolol injection. Decrease of dose or termination of infusion reverses hypotension, usually within 30 minutes.
Bradycardia
Bradycardia, including sinus pause, heart block, severe bradycardia, and cardiac arrest have occurred with the use of esmolol injection. Patients with first-degree atrioventricular block, sinus node dysfunction, or conduction disorders may be at increased risk. Monitor heart rate and rhythm in patients receiving esmolol.
If severe bradycardia develops, reduce or stop esmolol.
Cardiac Failure
Beta blockers, like esmolol injection, can cause depression of myocardial contractility and may precipitate heart failure and cardiogenic shock. At the first sign or symptom of impending cardiac failure, stop esmolol and start supportive therapy.
Intraoperative and Postoperative Tachycardia and/or Hypertension
Monitor vital signs closely and titrate esmolol slowly in the treatment of patients whose blood pressure is primarily driven by vasoconstriction associated with hypothermia.
Reactive Airways Disease
Patients with reactive airways disease should, in general, not receive beta blockers. Because of its relative beta1 selectivity and titratability, titrate esmolol to the lowest possible effective dose. In the event of bronchospasm, stop the infusion immediately; a beta2 stimulating agent may be administered with appropriate monitoring of ventricular rates.
Use in Patients with Diabetes Mellitus and Hypoglycemia
In patients with hypoglycemia, or diabetic patients (especially those with labile diabetes) who are receiving insulin or other hypoglycemic agents, beta blockers may mask tachycardia occurring with hypoglycemia, but other manifestations such as dizziness and sweating may not be masked.
Concomitant use of beta blockers and antidiabetic agents can enhance the effect of antidiabetic agents (blood glucose–lowering).
Infusion Site Reactions
Infusion site reactions have occurred with the use of esmolol injection. They include irritation, inflammation, and severe reactions (thrombophlebitis, necrosis, and blistering), in particular when associated with extravasation. Avoid infusions into small veins or through a butterfly catheter.
If a local infusion site reaction develops, use an alternative infusion site and avoid extravasation.
Use in Patients with Prinzmetal's Angina
Beta blockers may exacerbate anginal attacks in patients with Prinzmetal’s angina because of unopposed alpha receptor–mediated coronary artery vasoconstriction. Do not use nonselective beta blockers.
Use in Patients with Pheochromocytoma
If esmolol is used in the setting of pheochromocytoma, give it in combination with an alpha-blocker, and only after the alpha-blocker has been initiated. Administration of beta-blockers alone in the setting of pheochromocytoma has been associated with a paradoxical increase in blood pressure from the attenuation of beta-mediated vasodilation in skeletal muscle.
Use in Hypovolemic Patients
In hypovolemic patients, esmolol injection can attenuate reflex tachycardia and increase the risk of hypotension.
Use in Patients with Peripheral Circulatory Disorders
In patients with peripheral circulatory disorders (including Raynaud’s disease or syndrome, and peripheral occlusive vascular disease), esmolol may aggravate peripheral circulatory disorders.
Abrupt Discontinuation of esmolol Injection
Severe exacerbations of angina, myocardial infarction, and ventricular arrhythmias have been reported in patients with coronary artery disease upon abrupt discontinuation of beta blocker therapy. Observe patients for signs of myocardial ischemia when discontinuing esmolol.
Heart rate increases moderately above pretreatment levels 30 minutes after esmolol discontinuation.
Hyperkalemia
Beta blockers, including esmolol, have been associated with increases in serum potassium levels and hyperkalemia. The risk is increased in patients with risk factors such as renal impairment. Intravenous administration of beta blockers has been reported to cause potentially life-threatening hyperkalemia in hemodialysis patients. Monitor serum electrolytes during therapy with esmolol.
Use in Patients with Metabolic Acidosis
Beta blockers, including esmolol, have been reported to cause hyperkalemic renal tubular acidosis. Acidosis in general may be associated with reduced cardiac contractility.
Use in Patients with Hyperthyroidism
Beta-adrenergic blockade may mask certain clinical signs (e.g., tachycardia) of hyperthyroidism. Abrupt withdrawal of beta blockade might precipitate a thyroid storm; therefore, monitor patients for signs of thyrotoxicosis when withdrawing beta blocking therapy.
Use in Patients at Risk of Severe Acute Hypersensitivity Reactions
When using beta blockers, patients at risk of anaphylactic reactions may be more reactive to allergen exposure (accidental, diagnostic, or therapeutic).
Patients using beta blockers may be unresponsive to the usual doses of epinephrine used to treat anaphylactic or anaphylactoid reactions.
Adverse Reactions
Clinical Trials Experience
Clinical Trials Experience
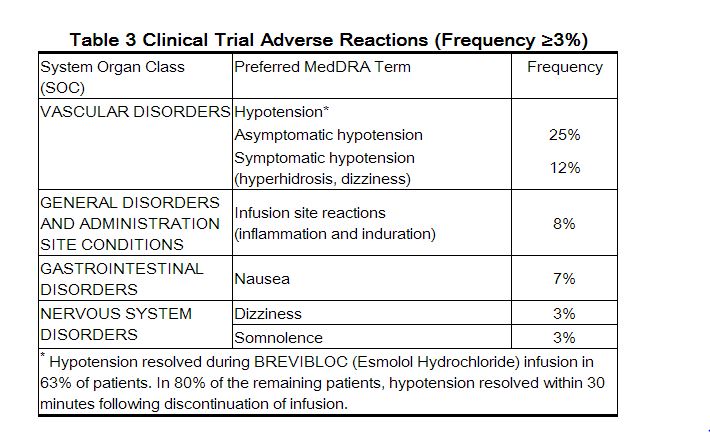
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- The following adverse reaction rates are based on use of esmolol in clinical trials involving 369 patients with supraventricular tachycardia and over 600 intraoperative and postoperative patients enrolled in clinical trials. Most adverse effects observed in controlled clinical trial settings have been mild and transient. The most important and common adverse effect has been hypotension. Deaths have been reported in post-marketing experience occurring during complex clinical states where esmolol was presumably being used simply to control ventricular rate
Clinical Trial Adverse Reactions (Frequency <3%)
Psychiatric Disorders
- Confusional state and agitation (~2%)
- Anxiety, depression and abnormal thinking (<1%)
Nervous System Disorders
- Headache (~ 2%)
- Paresthesia, syncope, speech disorder, and lightheadedness (<1%)
- Convulsions (<1%), with one death
Vascular Disorders
- Peripheral ischemia (~1%)
- Pallor and flushing (<1%)
Gastrointestinal Disorders
- Vomiting (~1%)
- Dyspepsia, constipation, dry mouth, and abdominal discomfort (<1%)
Renal and Urinary Disorders
- Urinary retention (<1%)
Postmarketing Experience
In addition to the adverse reactions reported in clinical trials, the following adverse reactions have been reported in the post-marketing experience. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate reliably their frequency or to establish a causal relationship to drug exposure.
Cardiac Disorders
- Cardiac arrest, Coronary arteriospasm
Skin and Subcutaneous Tissue Disorders
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
(Description)
Labor and Delivery
(Description)
Nursing Mothers
(Description)
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
Intravenous
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
Esmolol was tested for compatibility with ten commonly used intravenous fluids at a final concentration of 10 mg esmolol hydrochloride per mL. was found to be compatible with the following solutions and was stable for at least 24 hours at controlled room temperature or under refrigeration:
- Dextrose (5%) Injection, USP
- Dextrose (5%) in Lactated Ringer’s Injection
- Dextrose (5%) in Ringer’s Injection
- Dextrose (5%) and Sodium Chloride (0.45%) Injection, USP
- Dextrose (5%) and Sodium Chloride (0.9%) Injection, USP
- Lactated Ringer’s Injection, USP
- Potassium Chloride (40 mEq/liter) in Dextrose (5%) Injection, USP
- Sodium Chloride (0.45%) Injection, USP
- Sodium Chloride (0.9%) Injection, USP
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
| |
Esmolol
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
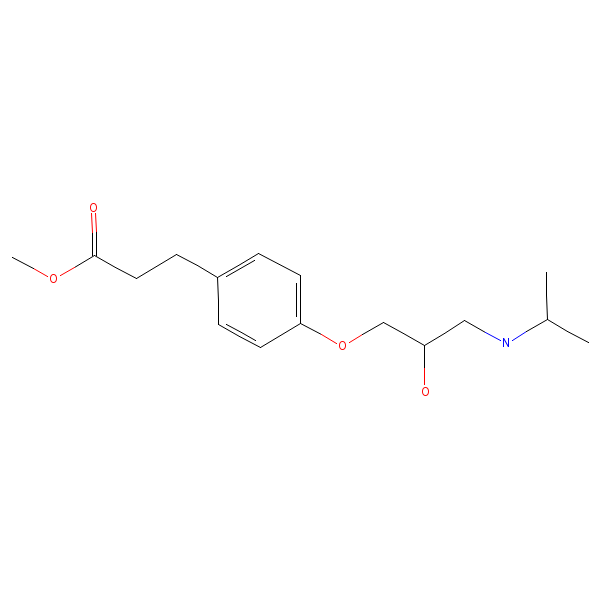
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
Storage
There is limited information regarding Esmolol Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Esmolol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Esmolol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-Esmolol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Esmolol Brand Names in the drug label.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.