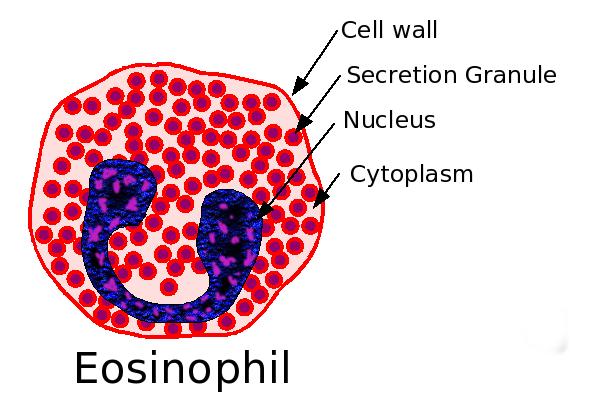
Eosinophil granulocyte
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Overview
Eosinophil granulocytes, usually called eosinophils (or, less commonly, acidophils), are white blood cells of the immune system that are responsible for combating infection and parasites in vertebrates. They also control mechanisms associated with allergy and asthma. They are granulocytes that develop in the bone marrow before migrating into blood.
These cells are eosinophilic or 'acid-loving': Normally transparent, they appear brick-red when stained with eosin, a dye, using the Romanowsky method. The staining is concentrated in small granules within the cellular cytoplasm, which contain many chemical mediators, such as histamine and proteins such as eosinophil peroxidase, RNase, DNases, lipase, plasminogen, and Major Basic Protein. These mediators are released by a process called degranulation following activation of the eosinophil, and are toxic to both parasite and host tissues.
Eosinophils make up about 1-6% of white blood cells, and are about 12-17 micrometers in size.[1] They are found in the medulla and the junction between the cortex and medulla of the thymus, and, in the lower gastrointestinal tract, ovary, uterus, spleen, and lymph nodes, but not in the lung, skin, esophagus, or some other internal organs under normal conditions. The presence of eosinophils in these latter organs is associated with disease. Eosinophils persist in the circulation for 8-12 hours, and can survive in tissue for an additional 8-12 days in the absence of stimulation.[2]
Eosinophil development, migration and activation
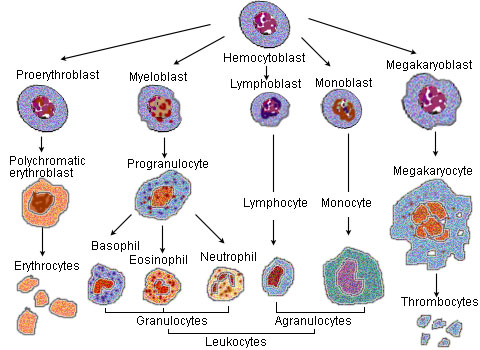
Eosinophils develop and mature in bone marrow. They differentiate from myeloid precursor cells in response to the cytokins interleukin 3 (IL-3), interleukin 5 (IL-5), and granulocyte macrophage colony-stimulating factor (GM-CSF).[3][4][5] Eosinophils produce and store many secondary granule proteins prior to their exit from the bone marrow. After maturation, eosinophils circulate in blood and migrate to inflammatory sites in tissues, or to sites of helminth infection in response to chemokines like CCL11 (eotaxin-1), CCL24 (eotaxin-2), CCL5 (RANTES), and certain leukotrienes like leukotriene B4 (LTB4). At these infectious sites, eosinophils are activated by Type 2 cytokines released from a specific subset of helper T cells (Th2); IL-5, GM-CSF, and IL-3 are important for eosinophil activation as well as maturation.
Functions of eosinophils
Following activation, eosinophils effector functions include production of:
- cationic granule proteins and their release by degranulation.[6]
- reactive oxygen species such as superoxide.[7]
- lipid mediators like the eicosanoids from the leukotriene (e.g., LTC4, LTD4, LTE4) and prostaglandin (e.g., PGE2) families.[8]
- enzymes, such as elastase
- growth factors such as TGF beta, VEGF, and PDGF.[9][10]
- cytokines such as IL-1, IL-2, IL-4, IL-5, IL-6, IL-8, IL-13, and TNF alpha.[11]
In addition, eosinophils play a role in fighting viral infections, which is evident from the abundance of RNAses they contain within their granules, and in fibrin removal during inflammation. Eosinophils are considered the main effector cells in allergic responses and asthma pathogenesis and are associated with disease severity. They also fight helminth (worm) colonization and may be slightly elevated in the presence of certain parasites. Eosinophils are also involved in many other biological processes, including postpubertal mammary gland development, oestrus cycling, allograft rejection and neoplasia.[11] They have also recently been implicated in antigen presentation to T cells.[12]
Eosinophil granule proteins
Following activation by an immune stimulus, eosinophils degranulate to release an array of cytotoxic granule cationic proteins that are capable of inducing tissue damage and dysfunction.[13] These include:
- Major basic protein (MBP)
- eosinophil cationic protein (ECP)
- eosinophil peroxidase (EPO)
- eosinophil-derived neurotoxin (EDN)
Major basic protein, eosinophil peroxidase, and eosinophil cationic protein are toxic to many tissues.[11] Eosinophil cationic protein and eosinophil-derived neurotoxin are ribonucleases with antiviral activity.[14] Major basic protein induces mast cell and basophil degranulation, and is implicated in peripheral nerve remodelling.[15][16] Eosinophil cationic protein creates toxic pores in the membranes of target cells allowing potential entry of other cytotoxic molecules to the cell,[17] can inhibit proliferation of T cells, suppress antibody production by B cells, induce degranulation by mast cells, and stimulate fibroblast cells to secrete mucus and glycosaminoglycan.[18] Eosinophil peroxidase forms reactive oxygen species and reactive nitrogen intermediates that promote oxidative stress in the target, causing cell death by apoptosis and necrosis.[11]
Eosinophilia
An increase in eosinophils, i.e., the presence of more than 500 eosinophils/microlitre of blood is called an eosinophilia, and is typically seen in people with a parasitic infestation of the intestines, a collagen vascular disease (such as rheumatoid arthritis), malignant diseases such as Hodgkin's Disease, extensive skin diseases (such as exfoliative dermatitis), Addison's Disease, in the squamous epithelium of the esophagus in the case of reflux esophagitis, and with the use of certain drugs such as penicillin and meropenem. In 1989, contaminated L-tryptophan supplements caused a deadly form of eosinophilia known as eosinophilia-myalgia syndrome.
Many infections case cause eosinophilia[19]
Hypereosinophilic syndromes (HES)
HES are defined as[20]:
- " AEC ≥ 1500/μL and clinical manifestations attributable to eosinophilia" OR
- "Tissue hypereosinophilia with blood eosinophilia (AEC above the upper limit of normal for the reference laboratory"
The six proposed HES are[20]:
- "myeloid hypereosinophilic syndrome (MHES)—HES with presumed or proven clonal eosinophilia"
- "lymphocytic variant hypereosinophilic syndrome (LHES)—HES with an aberrant or clonal lymphocyte population driving the eosinophilia"
- "overlap HES—eosinophilic disease restricted to a single organ system or defined eosinophilic syndromes that overlap in presentation with idiopathic HES (eg, EGPA)"
- "associated HES—HES in the setting of a known secondary cause that requires treatment directed at the cause rather than the eosinophilia itself (eg, helminth infection, drug hypersensitivity, and immunodeficiency)"
- "familial HES"
- "idiopathic HES—HES that does not fall into any of the other categories"
Eosinopenia
Eosinopenia is a decrease in eosinophil number, which occurs when glucocorticoids are administered or when Cushing's disease is present. Dr. Harvey Cushing, the man who discovered the disease, identified eosinopenia as one of the primary indicators in a patient suffering that disease. Over the years, with the increase in glucocorticoid therapy and the growing stresses in our society (another cause of a suppressed count), Eosinopenia has lost favor as a Cushing's diagnostic tool. That fact causes many people suffering Cushing's to often go undiagnosed for years until their symptoms become more severe.
Treatment
Treatments used to combat eosinophils include:
- monoclonal antibody therapy against IL-5 - promote apoptosis
- antagonists of leukotriene synthesis or receptors
- corticosteroids- promote apoptosis
- Gleevec (STI571)- inhibits PDGF-BB in hypereosinophilic leukemia
Additional images
-
Blood cell lineage
-
Eosinophil
-
Eosinophil
References
- ↑ Young, Barbara; Lowe, joseph o'connell; Stevens, Alan; Heath, John W. (2006), Wheater's Functional Histology (5 ed.), Elsevier Limited, ISBN 0-443-06850-X
- ↑ Young, Barbara; Lowe, James S.; Stevens, Alan; Heath, John W. (2006), Wheater's Functional Histology (5 ed.), Elsevier Limited, ISBN 0-443-06850-X
- ↑ Metcalf D, Begley C, Nicola N, Johnson G (1987). "Quantitative responsiveness of murine hemopoietic populations in vitro and in vivo to recombinant multi-CSF (IL-3)". Exp Hematol. 15 (3): 288–95. PMID 3493174.
- ↑ Metcalf D, Burgess A, Johnson G, Nicola N, Nice E, DeLamarter J, Thatcher D, Mermod J (1986). "In vitro actions on hemopoietic cells of recombinant murine GM-CSF purified after production in Escherichia coli: comparison with purified native GM-CSF". J Cell Physiol. 128 (3): 421–31. PMID 3528176.
- ↑ Yamaguchi Y, Suda T, Suda J, Eguchi M, Miura Y, Harada N, Tominaga A, Takatsu K (1988). "Purified interleukin 5 supports the terminal differentiation and proliferation of murine eosinophilic precursors". J Exp Med. 167 (1): 43–56. PMID 3257253.
- ↑ Trulson A, Byström J, Engström A, Larsson R, Venge P (2007). "The functional heterogeneity of eosinophil cationic protein is determined by a gene polymorphism and post-translational modifications". Clin Exp Allergy. 37 (2): 208–18. PMID 17250693.
- ↑ Saito K, Nagata M, Kikuchi I, Sakamoto Y (2004). "Leukotriene D4 and eosinophil transendothelial migration, superoxide generation, and degranulation via beta2 integrin". Ann Allergy Asthma Immunol. 93 (6): 594–600. PMID 15609771.
- ↑ Bandeira-Melo C, Bozza P, Weller P (2002). "The cellular biology of eosinophil eicosanoid formation and function". J Allergy Clin Immunol. 109 (3): 393–400. PMID 11897981.
- ↑ Kato Y, Fujisawa T, Nishimori H, Katsumata H, Atsuta J, Iguchi K, Kamiya H. "Leukotriene D4 induces production of transforming growth factor-beta1 by eosinophils". Int Arch Allergy Immunol. 137 Suppl 1: 17–20. PMID 15947480.
- ↑ Horiuchi T, Weller P (1997). "Expression of vascular endothelial growth factor by human eosinophils: upregulation by granulocyte macrophage colony-stimulating factor and interleukin-5". Am J Respir Cell Mol Biol. 17 (1): 70–7. PMID 9224211.
- ↑ 11.0 11.1 11.2 11.3 Rothenberg M, Hogan S. "The eosinophil". Annu Rev Immunol. 24: 147–74. PMID 16551246.
- ↑ Shi H (2004). "Eosinophils function as antigen-presenting cells". J Leukoc Biol. 76 (3): 520–7. PMID 15218055.
- ↑ Gleich G, Adolphson C. "The eosinophilic leukocyte: structure and function". Adv Immunol. 39: 177–253. PMID 3538819.
- ↑ Slifman N, Loegering D, McKean D, Gleich G (1986). "Ribonuclease activity associated with human eosinophil-derived neurotoxin and eosinophil cationic protein". J Immunol. 137 (9): 2913–7. PMID 3760576.
- ↑ Zheutlin L, Ackerman S, Gleich G, Thomas L (1984). "Stimulation of basophil and rat mast cell histamine release by eosinophil granule-derived cationic proteins". J Immunol. 133 (4): 2180–5. PMID 6206154.
- ↑ Morgan R, Costello R, Durcan N, Kingham P, Gleich G, McLean W, Walsh M (2005). "Diverse effects of eosinophil cationic granule proteins on IMR-32 nerve cell signaling and survival". Am J Respir Cell Mol Biol. 33 (2): 169–77. PMID 15860794.
- ↑ Young J, Peterson C, Venge P, Cohn Z. "Mechanism of membrane damage mediated by human eosinophil cationic protein". Nature. 321 (6070): 613–6. PMID 2423882.
- ↑ Venge P, Byström J, Carlson M, Hâkansson L, Karawacjzyk M, Peterson C, Sevéus L, Trulson A (1999). "Eosinophil cationic protein (ECP): molecular and biological properties and the use of ECP as a marker of eosinophil activation in disease". Clin Exp Allergy. 29 (9): 1172–86. PMID 10469025.
- ↑ NEJM 20223 doi:10.1056/NEJMcpc2201248
- ↑ 20.0 20.1 Klion A (2018). "Hypereosinophilic syndrome: approach to treatment in the era of precision medicine". Hematology Am Soc Hematol Educ Program. 2018 (1): 326–331. doi:10.1182/asheducation-2018.1.326. PMC 6245960. PMID 30504328.