Enoxaparin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
SPINAL/EPIDURAL HEMATOMA
See full prescribing information for complete Boxed Warning.
* Epidural or spinal hematomas may occur in patients who are anticoagulated with low molecular weight heparins (LMWH) or heparinoids and are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
|
Overview
Enoxaparin is an anticoagulant and low molecular weight heparin that is FDA approved for the treatment of acute deep vein thrombosis, acute ST-segment elevation myocardial infarction and prophylaxis of deep vein thrombosis and ischemic complications of unstable angina and non-Q-wave myocardial infarction. There is a Black Box Warning for this drug as shown here. Common adverse reactions include diarrhea, nausea, anemia, major bleeding, thrombocytopenia, increased liver function test, and fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Prophylaxis of Deep Vein Thrombosis
In patients undergoing abdominal surgery who are at risk for thromboembolic complications
- Dosing information
- Recommended dose: 40 mg SC q24h (with the initial dose given 2 hours prior to surgery)
- Duration of administration: 7 to 10 days (maximum: 12 days)
In patients undergoing hip or knee replacement surgery, during and following hospitalization
- Dosing information
- Recommended dose: 30 mg SC q12h (with the initial dose given 12 to 24 hours after surgery)
- Duration of administration: 7 to 10 days (maximum: 14 days)
- Alternative dosage for hip replacement surgery
- Recommended dose: 40 mg SC q24h (with the initial dose given 12±3 hours prior to surgery)
- Duration of administration: 3 weeks
In medical patients who are at risk for thromboembolic complications due to severely restricted mobility during acute illness
- Dosing information
- Recommended dose: 40 mg SC q24h
- Duration of administration: 6 to 11 days (maximum: 14 days)
Treatment of Acute Deep Vein Thrombosis (With or Without Pulmonary Embolism)
- Dosing information
- Recommended dose:
- For outpatient: 1 mg/kg SC q12h
- For inpatient: 1 mg/kg SC q12h or 1.5 mg/kg SC q24h
- Warfarin therapy should be initiated when appropriate (usually within 72 hours of enoxaparin injection).
- Duration of administration: 7 days (maximum: 17 days)
- Enoxaparin should be continued for a minimum of 5 days and until a therapeutic oral anticoagulant effect has been achieved (INR 2.0–3.0).
Prophylaxis of Ischemic Complications of Unstable Angina and Non-Q-Wave Myocardial Infarction
- Dosing information
- Recommended dose: 1 mg/kg SC q12h (in conjunction with aspirin 100–325 mg PO qd)
- Duration of administration: 2 to 8 days (maximum: 12.5 days)
Treatment of Acute ST-Segment Elevation Myocardial Infarction
- Dosing information
- Recommended dose (<75 years of age): 30 mg IV bolus PLUS 1 mg/kg SC for one dose FOLLOWED BY 1 mg/kg SC q12h
- Recommended dose (≥75 years of age): 0.75 mg/kg SC q12h
- All STEMI patients should receive aspirin 75–325 mg PO qd unless contraindicated.
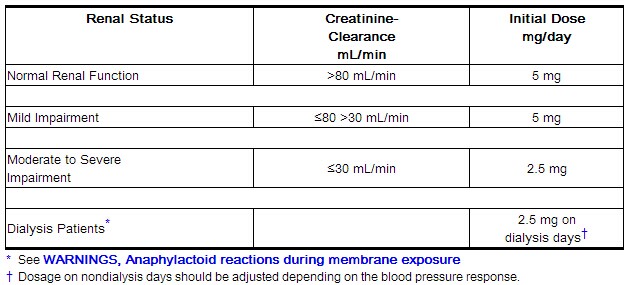
Renal Impairment
Although no dose adjustment is recommended in patients with moderate (creatinine clearance 30–50 mL/min) and mild (creatinine clearance 50–80 mL/min) renal impairment, all such patients should be observed carefully for signs and symptoms of bleeding.
The recommended prophylaxis and treatment dosage regimens for patients with severe renal impairment (creatinine clearance <30 mL/min) are described in Table 1.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Patients with solid tumor and low bleeding risk
- Developed by: American College of Chest Physicians (ACCP)
- Class of Recommendation: Class II
- Strength of Evidence: Level B
- Dosing Information
- Not applicable
Vitamin K Antagonists
- Developed by: American College of Chest Physicians (ACCP)
- Class of Recommendation: Grade 2
- Strength of Evidence: Level C
- Dosing Information
- Not applicable
Outpatient Cancer Patients
- Developed by: American College of Chest Physicians (ACCP)
- Class of Recommendation: Grade 2
- Strength of Evidence: Level B
- Dosing Information
- Not applicable
Thoracic Surgery
- Developed by: American College of Chest Physicians (ACCP)
- Class of Recommendation: Grade 2
- Strength of Evidence: Level B
- Dosing Information
- Not applicable
Spinal Surgery
- Developed by: American College of Chest Physicians (ACCP)
- Class of Recommendation: Grade 2
- Strength of Evidence: Level C
- Dosing Information
- Not applicable
Major Trauma
- Developed by: American College of Chest Physicians (ACCP)
- Class of Recommendation: Grade 2
- Strength of Evidence: Level C
- Dosing Information
- Not applicable
Management of HIT
- Developed by: American College of Chest Physicians (ACCP)
- Class of Recommendation: Grade 1
- Strength of Evidence: Level C
- Dosing Information
- Not applicable
Management of Acute HIT or Subacute HIT
- Developed by: American College of Chest Physicians (ACCP)
- Class of Recommendation: Grade 2
- Strength of Evidence: Level C
- Dosing Information
- Not applicable
Management in Patients with a Past History of HIT
- Developed by: American College of Chest Physicians (ACCP)
- Class of Recommendation: Grade 2
- Strength of Evidence: Level C
- Dosing Information
- Not applicable
Atrial Fibrillation Undergoing Cardioversion (≥48 h)
- Developed by: American College of Chest Physicians (ACCP)
- Class of Recommendation: Grade 1
- Strength of Evidence: Level B
- Dosing Information
- Not applicable
Atrial Fibrillation Undergoing Cardioversion(<48 h)
- Developed by: American College of Chest Physicians (ACCP)
- Class of Recommendation: Grade 2
- Strength of Evidence: Level C
- Dosing Information
- Not applicable
Endocarditis
- Developed by: American College of Chest Physicians (ACCP)
- Class of Recommendation: Grade 2
- Strength of Evidence: Level C
- Dosing Information
- Not applicable
Mechanical Prosthetic Heart Valves
- Developed by: American College of Chest Physicians (ACCP)
- Class of Recommendation: Grade 2
- Strength of Evidence: Level C
- Dosing Information
- Not applicable
VTE Prevention in Intracranial hemorrhage
Non–Guideline-Supported Use
Adjunction treatment of Cardiopulmonary bypass operation
- Dosing information
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information about the FDA-labeled indications and dosage information for children.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Homozygous Protein C Deficiency (Neonate)
- Developed by: American College of Chest Physicians (ACCP)
- Class of Recommendation: Class I
- Strength of Evidence: Level C
- Dosing Information
- For children less than 2 months: 1.5 mg/kg i.M bid
- For children over 2 months: 1 mg/kg i.M bid
Cerebral Sinovenous Thrombosis (Neonate)
- Developed by: American College of Chest Physicians (ACCP)
- Class of Recommendation: Class I
- Strength of Evidence: Level C
- Dosing Information
- For children less than 2 months: 1.5 mg/kg i.M bid
- For children over 2 months: 1 mg/kg i.M bid
Acute Ischemic Stroke (secondary to non-Moyamoya vasculopathy)
- Developed by: American College of Chest Physicians (ACCP)
- Class of Recommendation: Class I
- Strength of Evidence: Level C
- Dosing Information(Used as a initial treatment for at least 3 months)
- For children less than 2 months: 1.5 mg/kg i.M bid
- For children over 2 months: 1 mg/kg i.M bid
Ventricular assist device
Non–Guideline-Supported Use
- There is limited information about Off-Label Non–Guideline-Supported Use of Enoxaparin in pediatric patients.
Contraindications
- Active major bleeding
- Thrombocytopenia associated with a positive in vitro test for anti-platelet antibody in the presence of enoxaparin sodium
- Known hypersensitivity to enoxaparin sodium (e.g., pruritus, urticaria, anaphylactic/anaphylactoid reactions).
- Known hypersensitivity to heparin or pork products
- Known hypersensitivity to benzyl alcohol (which is in only the multi-dose formulation of Lovenox)
Warnings
SPINAL/EPIDURAL HEMATOMA
See full prescribing information for complete Boxed Warning.
* Epidural or spinal hematomas may occur in patients who are anticoagulated with low molecular weight heparins (LMWH) or heparinoids and are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
- Cases of epidural or spinal hemorrhage and subsequent hematomas have been reported with the use of Lovenox and epidural or spinal anesthesia/analgesia or spinal puncture procedures, resulting in long-term or permanent paralysis. The risk of these events is higher with the use of post-operative indwelling epidural catheters, with the concomitant use of additional drugs affecting hemostasis such as NSAIDs, with traumatic or repeated epidural or spinal puncture, or in patients with a history of spinal surgery or spinal deformity.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Enoxaparin Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Enoxaparin Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Enoxaparin Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Enoxaparin in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Enoxaparin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Enoxaparin during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Enoxaparin in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Enoxaparin in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Enoxaparin in geriatric settings.
Gender
There is no FDA guidance on the use of Enoxaparin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Enoxaparin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Enoxaparin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Enoxaparin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Enoxaparin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Enoxaparin in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Enoxaparin Administration in the drug label.
Monitoring
There is limited information regarding Enoxaparin Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Enoxaparin and IV administrations.
Overdosage
There is limited information regarding Enoxaparin overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Enoxaparin Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Enoxaparin Mechanism of Action in the drug label.
Structure
There is limited information regarding Enoxaparin Structure in the drug label.
Pharmacodynamics
There is limited information regarding Enoxaparin Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Enoxaparin Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Enoxaparin Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Enoxaparin Clinical Studies in the drug label.
How Supplied
There is limited information regarding Enoxaparin How Supplied in the drug label.
Storage
There is limited information regarding Enoxaparin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Enoxaparin
|?Pill Name
|?Drug Name
|?Pill Ingred
|?Pill Imprint
|?Pill Dosage
|?Pill Color
|?Pill Shape
|?Pill Size (mm)
|?Pill Scoring
|?NDC
|?Drug Author
|format=template
|template=DrugPageImages
|mainlabel=-
|sort=Pill Name
}}
Package and Label Display Panel
{{#ask: Label Page::Enoxaparin
|?Label Name
|format=template
|template=DrugLabelImages
|mainlabel=-
|sort=Label Page
}}
Patient Counseling Information
There is limited information regarding Enoxaparin Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Enoxaparin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Enoxaparin Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Enoxaparin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- For children less than 2 months: 1.5 mg/kg i.M bid
- For children over 2 months: 1 mg/kg i.M bid
- For children less than 2 months: 1.5 mg/kg i.M bid
- For children over 2 months: 1 mg/kg i.M bid
- For children less than 2 months: 1.5 mg/kg i.M bid
- For children over 2 months: 1 mg/kg i.M bid
Non–Guideline-Supported Use
- There is limited information about Off-Label Non–Guideline-Supported Use of Enoxaparin in pediatric patients.
Contraindications
- Active major bleeding
- Thrombocytopenia associated with a positive in vitro test for anti-platelet antibody in the presence of enoxaparin sodium
- Known hypersensitivity to enoxaparin sodium (e.g., pruritus, urticaria, anaphylactic/anaphylactoid reactions).
- Known hypersensitivity to heparin or pork products
- Known hypersensitivity to benzyl alcohol (which is in only the multi-dose formulation of Lovenox)
Warnings
|
SPINAL/EPIDURAL HEMATOMA
See full prescribing information for complete Boxed Warning.
* Epidural or spinal hematomas may occur in patients who are anticoagulated with low molecular weight heparins (LMWH) or heparinoids and are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
|
- Cases of epidural or spinal hemorrhage and subsequent hematomas have been reported with the use of Lovenox and epidural or spinal anesthesia/analgesia or spinal puncture procedures, resulting in long-term or permanent paralysis. The risk of these events is higher with the use of post-operative indwelling epidural catheters, with the concomitant use of additional drugs affecting hemostasis such as NSAIDs, with traumatic or repeated epidural or spinal puncture, or in patients with a history of spinal surgery or spinal deformity.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Enoxaparin Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Enoxaparin Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Enoxaparin Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Enoxaparin in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Enoxaparin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Enoxaparin during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Enoxaparin in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Enoxaparin in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Enoxaparin in geriatric settings.
Gender
There is no FDA guidance on the use of Enoxaparin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Enoxaparin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Enoxaparin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Enoxaparin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Enoxaparin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Enoxaparin in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Enoxaparin Administration in the drug label.
Monitoring
There is limited information regarding Enoxaparin Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Enoxaparin and IV administrations.
Overdosage
There is limited information regarding Enoxaparin overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Enoxaparin Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Enoxaparin Mechanism of Action in the drug label.
Structure
There is limited information regarding Enoxaparin Structure in the drug label.
Pharmacodynamics
There is limited information regarding Enoxaparin Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Enoxaparin Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Enoxaparin Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Enoxaparin Clinical Studies in the drug label.
How Supplied
There is limited information regarding Enoxaparin How Supplied in the drug label.
Storage
There is limited information regarding Enoxaparin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Enoxaparin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Enoxaparin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Enoxaparin Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Enoxaparin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Enoxaparin Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Enoxaparin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.