Emtricitabine: Difference between revisions
Gloria Picoy (talk | contribs) No edit summary |
Gloria Picoy (talk | contribs) No edit summary |
||
| Line 29: | Line 29: | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Emtricitabine in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Emtricitabine in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Emtricitabine in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Emtricitabine in pediatric patients. | ||
|contraindications=* In patients with previously demonstrated hypersensitivity to any of the components of the products. | |||
|warnings======Lactic Acidosis/Severe Hepatomegaly with Steatosis===== | |||
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs alone or in combination, including emtricitabine and other antiretrovirals. A majority of these cases have been in women. Obesity and prolonged nucleoside exposure may be risk factors. Particular caution should be exercised when administering nucleoside analogs to any patient with known risk factors for liver diseases; however, cases have also been reported in patients with no known risk factors. Treatment with EMTRIVA should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations). | |||
=====Patients Coinfected with HIV-1 and HBV===== | |||
It is recommended that all patients with HIV-1 be tested for the presence of chronic Hepatitis B virus (HBV) before initiating antiretroviral therapy. EMTRIVA is not approved for the treatment of chronic HBV infection and the safety and efficacy of EMTRIVA have not been established in patients coinfected with HBV and HIV-1. Severe acute exacerbations of Hepatitis B have been reported in patients after the discontinuation of EMTRIVA. In some patients infected with HBV and treated with EMTRIVA, the exacerbations of hepatitis B were associated with liver decompensation and liver failure. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who are coinfected with HIV-1 and HBV and discontinue EMTRIVA. If appropriate, initiation of anti-Hepatitis B therapy may be warranted. | |||
=====Coadministration with Related Products===== | |||
EMTRIVA is a component of ATRIPLA (a fixed-dose combination of efavirenz, emtricitabine, and tenofovir disoproxil fumarate), COMPLERA (a fixed-dose combination of emtricitabine, rilpivirine, and tenofovir disoproxil fumarate), STRIBILD (a fixed-dose combination of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate), and TRUVADA (a fixed-dose combination of emtricitabine and tenofovir disoproxil fumarate). Emtricitabine should not be coadministered with ATRIPLA, COMPLERA, STRIBILD, or TRUVADA. Due to similarities between emtricitabine and lamivudine, EMTRIVA should not be coadministered with other drugs containing lamivudine, including Combivir (lamivudine/zidovudine), Epivir or Epivir-HBV (lamivudine), Epzicom (abacavir sulfate/lamivudine), or Trizivir (abacavir sulfate/lamivudine/zidovudine). | |||
=====New Onset or Worsening Renal Impairment===== | |||
Emtricitabine is principally eliminated by the kidney. Reduction of the dosage of EMTRIVA is recommended for patients with impaired renal function. | |||
=====Fat Redistribution===== | |||
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established. | |||
=====Immune Reconstitution Syndrome===== | |||
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including EMTRIVA. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections [such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia (PCP), or tuberculosis], which may necessitate further evaluation and treatment. | |||
Autoimmune disorders (such as Graves' disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution, however, the time to onset is more variable, and can occur many months after initiation of treatment. | |||
|drugInteractions=The potential for drug interactions with emtricitabine has been studied in combination with zidovudine, indinavir, stavudine, famciclovir, and tenofovir disoproxil fumarate. There were no clinically significant drug interactions for any of these drugs. Drug interactions trials are described elsewhere in the labeling. | |||
|FDAPregCat=B | |||
|useInPregnancyFDA=The incidence of fetal variations and malformations was not increased in embryofetal toxicity studies performed with emtricitabine in mice at exposures (AUC) approximately 60-fold higher and in rabbits at approximately 120-fold higher than human exposures at the recommended daily dose. There are, however, no adequate and well-controlled trials in pregnant women. Because animal reproduction studies are not always predictive of human response, EMTRIVA should be used during pregnancy only if clearly needed. | |||
|AUSPregCat=B1 | |||
|useInNursing=The Centers for Disease Control and Prevention recommend that HIV-1-infected mothers not breast-feed their infants to avoid risking postnatal transmission of HIV-1. Samples of breast milk obtained from five HIV-1 infected mothers show that emtricitabine is secreted in human milk. Breastfeeding infants whose mothers are being treated with emtricitabine may be at risk for developing viral resistance to emtricitabine. Other emtricitabine-associated risks in infants breastfed by mothers being treated with emtricitabine are unknown. Because of both the potential for HIV-1 transmission and the potential for serious adverse reactions in nursing infants, mothers should be instructed not to breast-feed if they are receiving EMTRIVA. | |||
|useInPed=The safety and efficacy of emtricitabine in patients between 3 months and 21 years of age is supported by data from three open-label, non-randomized clinical trials in which emtricitabine was administered to 169 HIV-1 infected treatment-naive and experienced (defined as virologically suppressed on a lamivudine containing regimen for which emtricitabine was substituted for lamivudine) subjects. | |||
The pharmacokinetics of emtricitabine were studied in 20 neonates born to HIV-1-positive mothers. All neonates were HIV-1 negative at the end of the trial; the efficacy of emtricitabine in preventing or treating HIV-1 could not be determined. | |||
|useInGeri=Clinical trials of EMTRIVA did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger subjects. In general, dose selection for the elderly patient should be cautious, keeping in mind the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. | |||
|useInRenalImpair=It is recommended that the dose or dosing interval for EMTRIVA be modified in patients with creatinine clearance less than 50 mL/min or in patients who require dialysis. | |||
|administration=Oral | |||
|overdose=There is no known antidote for EMTRIVA. Limited clinical experience is available at doses higher than the therapeutic dose of EMTRIVA. In one clinical pharmacology trial single doses of emtricitabine 1200 mg were administered to 11 subjects. No severe adverse reactions were reported. | |||
The effects of higher doses are not known. If overdose occurs the patient should be monitored for signs of toxicity, and standard supportive treatment applied as necessary. | |||
Hemodialysis treatment removes approximately 30% of the emtricitabine dose over a 3-hour dialysis period starting within 1.5 hours of emtricitabine dosing (blood flow rate of 400 mL/min and a dialysate flow rate of 600 mL/min). It is not known whether emtricitabine can be removed by peritoneal dialysis. | |||
|mechAction=Emtricitabine, a synthetic nucleoside analog of cytidine, is phosphorylated by cellular enzymes to form emtricitabine 5'-triphosphate. Emtricitabine 5'-triphosphate inhibits the activity of the HIV-1 reverse transcriptase by competing with the natural substrate deoxycytidine 5'-triphosphate and by being incorporated into nascent viral DNA which results in chain termination. Emtricitabine 5'-triphosphate is a weak inhibitor of mammalian DNA polymerase α, β, ε, and mitochondrial DNA polymerase γ. | |||
|structure=It has a molecular formula of C8H10FN3O3S and a molecular weight of 247.24. It has the following structural formula: | |||
[[File:Emtricitabine chemical structure.png|thumb|none|500px]] | |||
|alcohol=Alcohol-Emtricitabine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Emtricitabine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 19:15, 23 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS AND POST TREATMENT EXACERBATION OF HEPATITIS B
See full prescribing information for complete Boxed Warning.
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs alone or in combination with other antiretrovirals.
Emtricitabine is not approved for the treatment of chronic hepatitis B virus (HBV) infection and the safety and efficacy of EMTRIVA have not been established in patients coinfected with HBV and HIV-1. Severe acute exacerbations of hepatitis B have been reported in patients who have discontinued EMTRIVA. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who are coinfected with HIV-1 and HBV and discontinue EMTRIVA. If appropriate, initiation of anti-hepatitis B therapy may be warranted.
|
Overview
Emtricitabine is a nucleoside reverse transcriptase inhibitor that is FDA approved for the treatment of HIV-1 infection in combination with other antiretroviral agents. There is a Black Box Warning for this drug as shown here. Common adverse reactions include headache, diarrhea, nausea, fatigue, dizziness, depression, insomnia, abnormal dreams, rash, abdominal pain, asthenia, increased cough, rhinitis and skin hyperpigmentation (pediatric patients).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Emtricitabine is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection.
- Dosage:
- Capsules: one 200 mg capsule administered once daily orally.
- Oral solution: 240 mg (24 mL) administered once daily orally.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Emtricitabine in adult patients.
Non–Guideline-Supported Use
- Chronic type B viral hepatitis
- Dosage: 200 mg/day for 48 weeks [1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Emtricitabine is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection.
- Dosage in pediatric patients between 0–3 months of age
- Oral solution: 3 mg per kg administered once daily orally.
- Dosage in pediatric patients between 3 months through 17 years
- Oral solution: 6 mg per kg up to a maximum of 240 mg (24 mL) administered once daily orally.
- Capsules: for children weighing more than 33 kg who can swallow an intact capsule, one 200 mg capsule administered once daily orally.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Emtricitabine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Emtricitabine in pediatric patients.
Contraindications
- In patients with previously demonstrated hypersensitivity to any of the components of the products.
Warnings
|
WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS AND POST TREATMENT EXACERBATION OF HEPATITIS B
See full prescribing information for complete Boxed Warning.
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs alone or in combination with other antiretrovirals.
Emtricitabine is not approved for the treatment of chronic hepatitis B virus (HBV) infection and the safety and efficacy of EMTRIVA have not been established in patients coinfected with HBV and HIV-1. Severe acute exacerbations of hepatitis B have been reported in patients who have discontinued EMTRIVA. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who are coinfected with HIV-1 and HBV and discontinue EMTRIVA. If appropriate, initiation of anti-hepatitis B therapy may be warranted.
|
Lactic Acidosis/Severe Hepatomegaly with Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs alone or in combination, including emtricitabine and other antiretrovirals. A majority of these cases have been in women. Obesity and prolonged nucleoside exposure may be risk factors. Particular caution should be exercised when administering nucleoside analogs to any patient with known risk factors for liver diseases; however, cases have also been reported in patients with no known risk factors. Treatment with EMTRIVA should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
Patients Coinfected with HIV-1 and HBV
It is recommended that all patients with HIV-1 be tested for the presence of chronic Hepatitis B virus (HBV) before initiating antiretroviral therapy. EMTRIVA is not approved for the treatment of chronic HBV infection and the safety and efficacy of EMTRIVA have not been established in patients coinfected with HBV and HIV-1. Severe acute exacerbations of Hepatitis B have been reported in patients after the discontinuation of EMTRIVA. In some patients infected with HBV and treated with EMTRIVA, the exacerbations of hepatitis B were associated with liver decompensation and liver failure. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who are coinfected with HIV-1 and HBV and discontinue EMTRIVA. If appropriate, initiation of anti-Hepatitis B therapy may be warranted.
Coadministration with Related Products
EMTRIVA is a component of ATRIPLA (a fixed-dose combination of efavirenz, emtricitabine, and tenofovir disoproxil fumarate), COMPLERA (a fixed-dose combination of emtricitabine, rilpivirine, and tenofovir disoproxil fumarate), STRIBILD (a fixed-dose combination of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate), and TRUVADA (a fixed-dose combination of emtricitabine and tenofovir disoproxil fumarate). Emtricitabine should not be coadministered with ATRIPLA, COMPLERA, STRIBILD, or TRUVADA. Due to similarities between emtricitabine and lamivudine, EMTRIVA should not be coadministered with other drugs containing lamivudine, including Combivir (lamivudine/zidovudine), Epivir or Epivir-HBV (lamivudine), Epzicom (abacavir sulfate/lamivudine), or Trizivir (abacavir sulfate/lamivudine/zidovudine).
New Onset or Worsening Renal Impairment
Emtricitabine is principally eliminated by the kidney. Reduction of the dosage of EMTRIVA is recommended for patients with impaired renal function.
Fat Redistribution
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including EMTRIVA. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections [such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia (PCP), or tuberculosis], which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves' disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution, however, the time to onset is more variable, and can occur many months after initiation of treatment.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Emtricitabine Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Emtricitabine Postmarketing Experience in the drug label.
Drug Interactions
The potential for drug interactions with emtricitabine has been studied in combination with zidovudine, indinavir, stavudine, famciclovir, and tenofovir disoproxil fumarate. There were no clinically significant drug interactions for any of these drugs. Drug interactions trials are described elsewhere in the labeling.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B
The incidence of fetal variations and malformations was not increased in embryofetal toxicity studies performed with emtricitabine in mice at exposures (AUC) approximately 60-fold higher and in rabbits at approximately 120-fold higher than human exposures at the recommended daily dose. There are, however, no adequate and well-controlled trials in pregnant women. Because animal reproduction studies are not always predictive of human response, EMTRIVA should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS): B1
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Emtricitabine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Emtricitabine during labor and delivery.
Nursing Mothers
The Centers for Disease Control and Prevention recommend that HIV-1-infected mothers not breast-feed their infants to avoid risking postnatal transmission of HIV-1. Samples of breast milk obtained from five HIV-1 infected mothers show that emtricitabine is secreted in human milk. Breastfeeding infants whose mothers are being treated with emtricitabine may be at risk for developing viral resistance to emtricitabine. Other emtricitabine-associated risks in infants breastfed by mothers being treated with emtricitabine are unknown. Because of both the potential for HIV-1 transmission and the potential for serious adverse reactions in nursing infants, mothers should be instructed not to breast-feed if they are receiving EMTRIVA.
Pediatric Use
The safety and efficacy of emtricitabine in patients between 3 months and 21 years of age is supported by data from three open-label, non-randomized clinical trials in which emtricitabine was administered to 169 HIV-1 infected treatment-naive and experienced (defined as virologically suppressed on a lamivudine containing regimen for which emtricitabine was substituted for lamivudine) subjects.
The pharmacokinetics of emtricitabine were studied in 20 neonates born to HIV-1-positive mothers. All neonates were HIV-1 negative at the end of the trial; the efficacy of emtricitabine in preventing or treating HIV-1 could not be determined.
Geriatic Use
Clinical trials of EMTRIVA did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger subjects. In general, dose selection for the elderly patient should be cautious, keeping in mind the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Emtricitabine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Emtricitabine with respect to specific racial populations.
Renal Impairment
It is recommended that the dose or dosing interval for EMTRIVA be modified in patients with creatinine clearance less than 50 mL/min or in patients who require dialysis.
Hepatic Impairment
There is no FDA guidance on the use of Emtricitabine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Emtricitabine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Emtricitabine in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
There is limited information regarding Emtricitabine Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Emtricitabine and IV administrations.
Overdosage
There is no known antidote for EMTRIVA. Limited clinical experience is available at doses higher than the therapeutic dose of EMTRIVA. In one clinical pharmacology trial single doses of emtricitabine 1200 mg were administered to 11 subjects. No severe adverse reactions were reported.
The effects of higher doses are not known. If overdose occurs the patient should be monitored for signs of toxicity, and standard supportive treatment applied as necessary.
Hemodialysis treatment removes approximately 30% of the emtricitabine dose over a 3-hour dialysis period starting within 1.5 hours of emtricitabine dosing (blood flow rate of 400 mL/min and a dialysate flow rate of 600 mL/min). It is not known whether emtricitabine can be removed by peritoneal dialysis.
Pharmacology
There is limited information regarding Emtricitabine Pharmacology in the drug label.
Mechanism of Action
Emtricitabine, a synthetic nucleoside analog of cytidine, is phosphorylated by cellular enzymes to form emtricitabine 5'-triphosphate. Emtricitabine 5'-triphosphate inhibits the activity of the HIV-1 reverse transcriptase by competing with the natural substrate deoxycytidine 5'-triphosphate and by being incorporated into nascent viral DNA which results in chain termination. Emtricitabine 5'-triphosphate is a weak inhibitor of mammalian DNA polymerase α, β, ε, and mitochondrial DNA polymerase γ.
Structure
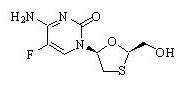
It has a molecular formula of C8H10FN3O3S and a molecular weight of 247.24. It has the following structural formula:
Pharmacodynamics
There is limited information regarding Emtricitabine Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Emtricitabine Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Emtricitabine Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Emtricitabine Clinical Studies in the drug label.
How Supplied
There is limited information regarding Emtricitabine How Supplied in the drug label.
Storage
There is limited information regarding Emtricitabine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Emtricitabine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Emtricitabine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Emtricitabine Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Emtricitabine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Emtricitabine Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Emtricitabine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Lim SG, Ng TM, Kung N, Krastev Z, Volfova M, Husa P, et al. (2006). "A double-blind placebo-controlled study of emtricitabine in chronic hepatitis B". Arch. Intern. Med. 166 (1): 49–56. doi:10.1001/archinte.166.1.49. PMID 16401810.
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [4]
Overview
Emtricitabine (FTC), with trade name Emtriva (formerly Coviracil), is a nucleoside reverse transcriptase inhibitor (NRTI) for the treatment of HIV infection in adults and children.
Emtricitabine is also marketed in a fixed-dose combination with tenofovir (Viread) under the brand name Truvada. A fixed-dose triple combination of emtricitabine, tenofovir and efavirenz (Sustiva, marketed by Bristol-Myers Squibb) was approved by the U.S. Food and Drug Administration (FDA) on July 12, 2006 under the brand name Atripla.
Emtricitabine makes up one fourth of the Quad pill (brand name: Stribild).
Category
Antiretroviral
US Brand Names
EMTRIVA®
FDA Package Insert
Description | Clinical Pharmacology | Microbiology | Indications and Usage | Contraindications | Warnings and Precautions | Adverse Reactions | Overdosage | Clinical Studies | Dosage and Administration | How Supplied | Labels and Packages
Historical Perspective
Emtricitabine was discovered by Dr. Dennis C. Liotta, Dr. Raymond F. Schinazi, and Dr. Woo-Baeg Choi of Emory University and licensed toTriangle Pharmaceuticals by Emory in 1996.[1] Triangle Pharmaceuticals was acquired in 2003 by Gilead Sciences, who completed development and now market the product with the brand name Emtriva.
It was approved by the FDA July 2, 2003. It is very similar to lamivudine (3TC) and cross-resistance between the two is near-universal.
Mechanism of Action
Emtricitabine is an analogue of cytidine which serves as a nucleoside reverse transcriptase inhibitor (NRTI). The drug becomes phosphorylated intracellularly to emtricitabine 5'-triphosphate and interferes with HIV RNA-dependent DNA polymerase resulting in inhibition of viral replication.
References
- ↑ Leaf, Clifton (September 19, 2005). "The Law of Unintended Consequences". CNN.