Diloxanide furoate: Difference between revisions
Gerald Chi (talk | contribs) mNo edit summary |
No edit summary |
||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Drugbox | |||
{{ | | Verifiedfields = changed | ||
| verifiedrevid = 470454750 | |||
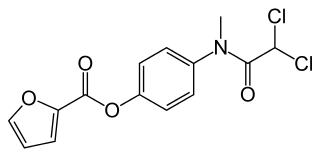
| IUPAC_name = 4-[(dichloroacetyl)(methyl)amino]phenyl furan-2-carboxylate | |||
| image = Diloxanide furoate.png | |||
| drug_name = Diloxanide | |||
== | <!--Clinical data--> | ||
| tradename = Furamide | |||
| Drugs.com = {{drugs.com|CONS|diloxanide}} | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = <!-- A / B / C / D / X --> | |||
| pregnancy_category = No available data | |||
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled--> | |||
| legal_CA = <!-- Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| legal_UK = <!-- GSL, P, POM, CD, or Class A, B, C --> | |||
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> | |||
| legal_status = Not approved <small>([[United States|US]], [[Canada|CA]])</small> | |||
| routes_of_administration = Oral | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = 90% (diloxanide) | |||
| protein_bound = | |||
| metabolism = [[hydrolysis|Hydrolyzed]] to furoic acid and diloxanide, which undergoes extensive [[glucuronidation]] | |||
| elimination_half-life = 3 hours | |||
| excretion = [[Kidney|Renal]] (90%), fecal (10%) | |||
== | <!--Identifiers--> | ||
| CAS_number_Ref = {{cascite|changed|??}} | |||
| CAS_number = 3736-81-0 | |||
| ATC_prefix = P01 | |||
| ATC_suffix = AC01 | |||
| PubChem = 19529 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB08792 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 18400 | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D02480 | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 1334860 | |||
<!--Chemical data--> | |||
| C=14 | H=11 | Cl=2 | N=1 | O=4 | |||
== | | molecular_weight = 328.147 g/mol | ||
| smiles = O=C(Oc1ccc(N(C(=O)C(Cl)Cl)C)cc1)c2occc2 | |||
| InChI = 1/C14H11Cl2NO4/c1-17(13(18)12(15)16)9-4-6-10(7-5-9)21-14(19)11-3-2-8-20-11/h2-8,12H,1H3 | |||
| InChIKey = BDYYDXJSHYEDGB-UHFFFAOYAB | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C14H11Cl2NO4/c1-17(13(18)12(15)16)9-4-6-10(7-5-9)21-14(19)11-3-2-8-20-11/h2-8,12H,1H3 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = BDYYDXJSHYEDGB-UHFFFAOYSA-N | |||
=== | }} | ||
__NOTOC__ | |||
{{SI}} | |||
{{CMG}} | |||
==Overview== | |||
'''Diloxanide furoate''' is a luminal amebicide used in the treatment of ''[[Amebiasis]].'' It is considered the luminal agent of choice for mild intestinal amebiasis or asymptomatic cyst carriers.It can also be added to metronidazole(active drug in luminal and extraintestinal amebiasis) in acute amebic dysentery as well as hepatic abscess(In hepatic abscess it is for the control of cysts in the lumen which may cause relapse). The drug was discovered by ''The Boots Company Plc'' in 1956 and introduced as ''Furamide''. The ''Furamide'' brand is now owned by [[Abbott Laboratories]]. It is not available in the US. In India it is available as ''Amicline'' by ''Franco-Indian''. | |||
= | |||
= | |||
= | |||
= | |||
= | |||
<!-- Society and culture --> | |||
It is on the [[World Health Organization's List of Essential Medicines]], a list of the most important medication needed in a basic [[health system]]. | |||
A [[ | ==Safety and effectiveness== | ||
A 13-year study conducted by the United States [[Center for Disease Control]] between 1977 and 1990 found that this drug had a low incidence of side effects and was successful in treatment of 86% of asymptomatic carriers of ''[[Entamoeba histolytica]].'' | |||
==References== | ==References== | ||
{{Reflist|2}} | {{Reflist|2}} | ||
[[Category: | [[Category:Antiprotozoal agents]] | ||
[[Category: | [[Category:Drug]] | ||
Latest revision as of 13:04, 23 April 2015
 | |
| Clinical data | |
|---|---|
| Trade names | Furamide |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 90% (diloxanide) |
| Metabolism | Hydrolyzed to furoic acid and diloxanide, which undergoes extensive glucuronidation |
| Elimination half-life | 3 hours |
| Excretion | Renal (90%), fecal (10%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C14H11Cl2NO4 |
| Molar mass | 328.147 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Diloxanide furoate is a luminal amebicide used in the treatment of Amebiasis. It is considered the luminal agent of choice for mild intestinal amebiasis or asymptomatic cyst carriers.It can also be added to metronidazole(active drug in luminal and extraintestinal amebiasis) in acute amebic dysentery as well as hepatic abscess(In hepatic abscess it is for the control of cysts in the lumen which may cause relapse). The drug was discovered by The Boots Company Plc in 1956 and introduced as Furamide. The Furamide brand is now owned by Abbott Laboratories. It is not available in the US. In India it is available as Amicline by Franco-Indian.
It is on the World Health Organization's List of Essential Medicines, a list of the most important medication needed in a basic health system.
Safety and effectiveness
A 13-year study conducted by the United States Center for Disease Control between 1977 and 1990 found that this drug had a low incidence of side effects and was successful in treatment of 86% of asymptomatic carriers of Entamoeba histolytica.
References
- Pages with script errors
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- Articles with changed CASNo identifier
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Infobox drug articles with non-default infobox title
- Articles without UNII source
- Drugboxes which contain changes to verified fields
- Antiprotozoal agents
- Drug