Dienestrol: Difference between revisions
m (Protected "Dienestrol": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
No edit summary |
||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{Drugbox | ||
| Verifiedfields = changed | |||
| Watchedfields = changed | |||
| verifiedrevid = 460784619 | |||
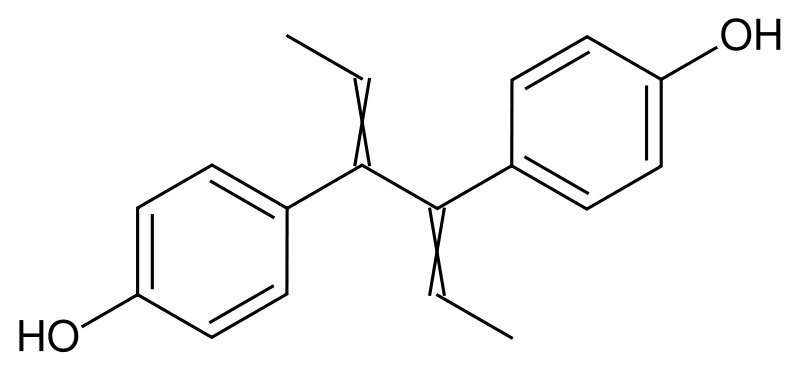
| IUPAC_name = 4-[4-(4-hydroxyphenyl)hexa-2,4-dien-3-yl]phenol; ''p''-[(''E,E'')-1-Ethylidene-2-(''p''-hydroxyphenyl)-2-butenyl]phenol | |||
| image = Dienestrol.svg | |||
<!--Clinical data--> | |||
| tradename = | |||
| Drugs.com = {{drugs.com|CONS|dienestrol}} | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = <!-- A / B / C / D / X --> | |||
| pregnancy_category = | |||
| legal_AU = <!-- Unscheduled / S2 / S4 / S8 --> | |||
| legal_UK = <!-- GSL / P / POM / CD --> | |||
| legal_US = <!-- OTC / Rx-only --> | |||
| legal_status = | |||
| routes_of_administration = | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = 50 to 80% | |||
| metabolism = | |||
| elimination_half-life = | |||
| excretion = | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| | | CAS_number_Ref = {{cascite|changed|??}} | ||
| CAS_number = 84-17-3 | | CAS_number = 84-17-3 | ||
| ATC_prefix = G03 | | ATC_prefix = G03 | ||
| ATC_suffix = CB01 | | ATC_suffix = CB01 | ||
| ATC_supplemental = {{ATC|G03|CC02}} | | ATC_supplemental = {{ATC|G03|CC02}} | ||
| PubChem = | | PubChem = 667476 | ||
| DrugBank = | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| C = 18 | H = 18 | O = 2 | | DrugBank = DB00890 | ||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 580857 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = RRW32X4U1F | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D00898 | |||
| ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| ChEBI = 4518 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 1018 | |||
<!--Chemical data--> | |||
| C=18 | H=18 | O=2 | |||
| molecular_weight = 266.334 g/mol | | molecular_weight = 266.334 g/mol | ||
| | | smiles = Oc2ccc(C(/C(c1ccc(O)cc1)=C/C)=C\C)cc2 | ||
| InChI = 1/C18H18O2/c1-3-17(13-5-9-15(19)10-6-13)18(4-2)14-7-11-16(20)12-8-14/h3-12,19-20H,1-2H3/b17-3+,18-4+ | |||
| | | InChIKey = NFDFQCUYFHCNBW-SCGPFSFSBL | ||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C18H18O2/c1-3-17(13-5-9-15(19)10-6-13)18(4-2)14-7-11-16(20)12-8-14/h3-12,19-20H,1-2H3/b17-3+,18-4+ | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | | StdInChIKey = NFDFQCUYFHCNBW-SCGPFSFSSA-N | ||
| | |||
| | |||
| | |||
| | |||
}} | }} | ||
__NOTOC__ | |||
{{SI}} | {{SI}} | ||
{{CMG}} | |||
==Overview== | |||
'''Dienestrol''' is a synthetic [[estrogen]]. | '''Dienestrol''' is a synthetic [[estrogen]]. | ||
{{ | |||
==Synthesis== | |||
[[File:Dienestrol.png|center|thumb|700px|Dienestrol synthesis:<ref>{{Cite doi|10.1098/rspb.1939.0015}}</ref>]] | |||
#[[Pinacol coupling]] of ''[[para isomer|p]]''-[[acetoxy]]-[[propiophenone]]. | |||
#[[Dehydration]] of the thus-obtained [[glycol]] with a mixture of [[acetyl chloride]] and [[acetic anhydride]] leads to the [[wikt:transoid|transoid]] [[diene]] system ([[bis]]-''β''-Methyl[[styrene]]-type). | |||
#[[Saponification]] removes the acetate groups and thus affords dienestrol. | |||
==See also== | |||
*[[Diethylstilbestrol]] | |||
==References== | |||
{{reflist|2}} | |||
[[Category:Synthetic estrogens]] | [[Category:Synthetic estrogens]] | ||
[[Category: | [[Category:Phenols]] | ||
[[Category:Drug]] | |||
Latest revision as of 13:37, 10 April 2015
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 50 to 80% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C18H18O2 |
| Molar mass | 266.334 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Dienestrol |
|
Articles |
|---|
|
Most recent articles on Dienestrol |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Dienestrol at Clinical Trials.gov Clinical Trials on Dienestrol at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Dienestrol
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Dienestrol Discussion groups on Dienestrol Patient Handouts on Dienestrol Directions to Hospitals Treating Dienestrol Risk calculators and risk factors for Dienestrol
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Dienestrol |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Dienestrol is a synthetic estrogen.
Synthesis
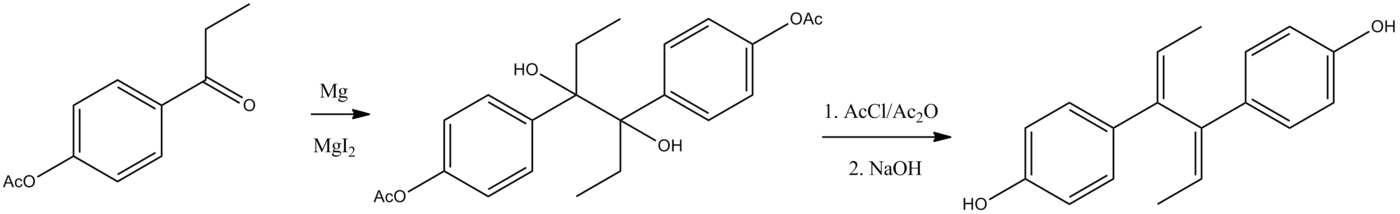
- Pinacol coupling of p-acetoxy-propiophenone.
- Dehydration of the thus-obtained glycol with a mixture of acetyl chloride and acetic anhydride leads to the transoid diene system (bis-β-Methylstyrene-type).
- Saponification removes the acetate groups and thus affords dienestrol.
See also
References
- Pages with script errors
- Template:drugs.com link with non-standard subpage
- Articles with changed CASNo identifier
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Synthetic estrogens
- Phenols
- Drug