Daptomycin description
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
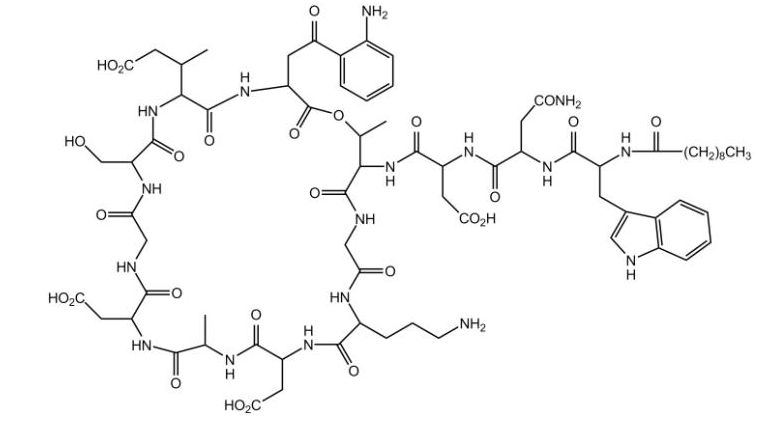
CUBICIN contains daptomycin, a cyclic lipopeptide antibacterial agent derived from the fermentation of Streptomyces roseosporus. The chemical name isN-decanoyl-L-tryptophyl-D-asparaginyl-L-aspartyl-L-threonylglycyl-L-ornithyl-L-aspartyl-D-alanyl-L-aspartylglycyl-D-seryl-threo-3-methyl-L-glutamyl-3-anthraniloyl-L-alanine ε1-lactone. The chemical structure is:
The empirical formula is C72H101N17O26; the molecular weight is 1620.67. CUBICIN is supplied in a single-use vial as a sterile, preservative-free, pale yellow to light brown, lyophilized cake containing approximately 500 mg of daptomycin for intravenous (IV) use following reconstitution with 0.9% sodium chloride injection [see Dosage and Administration (2.5)]. The only inactive ingredient is sodium hydroxide, which is used in minimal quantities for pH adjustment. Freshly reconstituted solutions of CUBICIN range in color from pale yellow to light brown.
References
http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a7975871-46a6-4e9b-a8b5-38bfcb465f0e