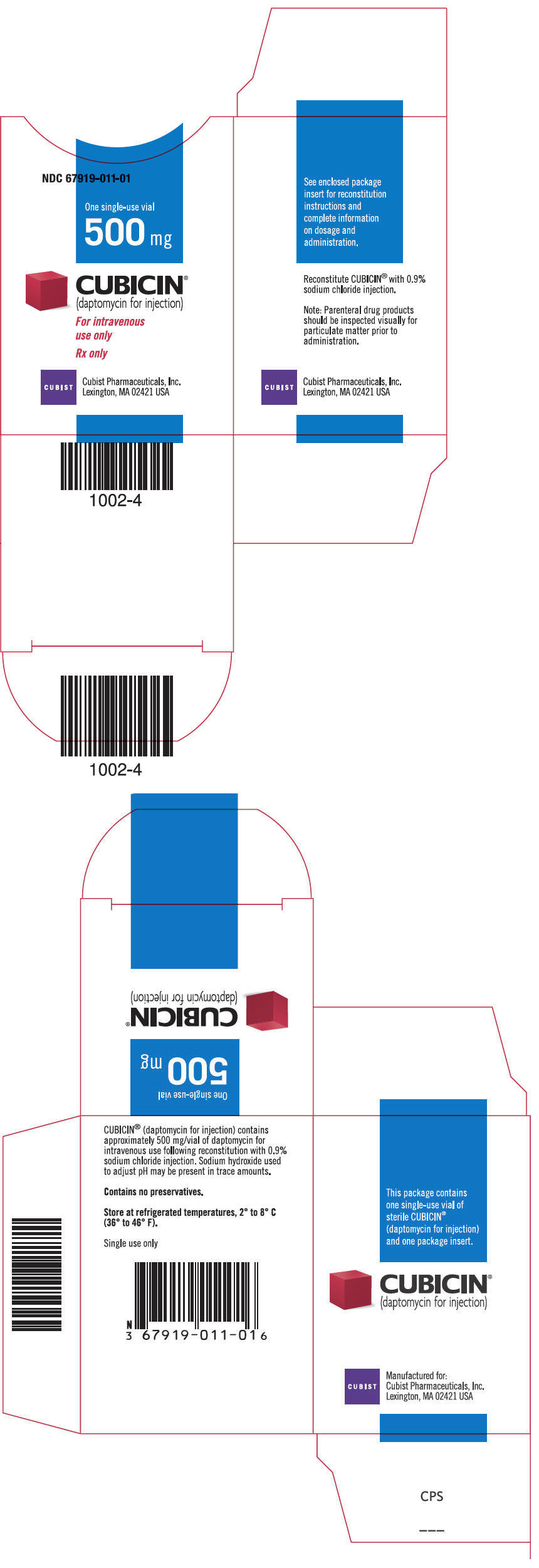
Daptomycin labels and packages
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
CUBICIN is a registered trademark of Cubist Pharmaceuticals, Inc. All other trademarks are property of their respective owners.
Manufactured for:
Cubist Pharmaceuticals, Inc. Lexington, MA 02421 USA
April 2013 (1004-12)
PRINCIPAL DISPLAY PANEL - 500 mg Vial 500 mg
CUBICIN® (daptomycin for injection)
For intravenous use only Single use only Rx only
 |
PRINCIPAL DISPLAY PANEL - 1 Vial Carton NDC 67919-011-01
One single-use vial 500 mg
CUBICIN® (daptomycin for injection)
For intravenous use only
Rx only
CUBIST Cubist Pharmaceuticals, Inc. Lexington, MA 02421 USA
 |
References
http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021572s033lbl.pdf