Cyclosporiasis laboratory findings: Difference between revisions
No edit summary |
|||
| Line 4: | Line 4: | ||
==Overview== | ==Overview== | ||
The diagnosis of cyclospora infection is confirmed examining stool specimens and several stool samples are require for a more precise identification of the oocysts. Laboratory techniques used to detect the cyclospora oocytes in stool include acid-fast staining, ultraviolet (UV) fluorescence microscope, and [[polymerase chain reaction]] (PCR) analysis.<ref>http://www.cdc.gov/parasites/cyclosporiasis/diagnosis.html</ref> | The diagnosis of [[cyclospora]] infection is confirmed examining [[stool]] specimens and several [[stool]] samples are require for a more precise identification of the oocysts. Laboratory techniques used to detect the cyclospora oocytes in stool include [[acid fast|acid-fast staining]], ultraviolet (UV) [[fluorescence microscope]], and [[polymerase chain reaction]] ([[PCR]]) analysis.<ref>http://www.cdc.gov/parasites/cyclosporiasis/diagnosis.html</ref> | ||
==Laboratory Findings== | ==Laboratory Findings== | ||
*Cyclospora infection is diagnosed by examining stool specimens. | *Cyclospora infection is diagnosed by examining [[stool]] specimens. | ||
*Laboratory testing for cyclospora is not routinely done in most U.S. laboratories, even when stool is tested for [[parasites]]. Therefore, if indicated, health care providers should specifically request testing for cyclospora. | *Laboratory testing for [[cyclospora]] is not routinely done in most U.S. laboratories, even when stool is tested for [[parasites]]. Therefore, if indicated, health care providers should specifically request testing for [[cyclospora]]. | ||
*Laboratory diagnosis can be difficult as the cyclospora [[oocysts]] may not be detected in stool, even if the patient is symptomatic. | *Laboratory diagnosis can be difficult as the [[cyclospora]] [[oocysts]] may not be detected in stool, even if the patient is symptomatic. | ||
*Therefore, patients might need to submit several specimens collected on different days. | *Therefore, patients might need to submit several specimens collected on different days. | ||
===Stool Examination=== | ===Stool Examination=== | ||
==== Wet Mount==== | ==== Wet Mount==== | ||
*In bright-field microscopy using differential interference contrast (DIC), oocysts appear as refractile spheres (8 to 10 μm) with a distinct oocyst wall, but may be confused with other objects. Under UV fluorescence microscopy, the oocyst wall autofluoresces. | *In bright-field microscopy using differential interference contrast (DIC), [[oocysts]] appear as refractile spheres (8 to 10 μm) with a distinct [[oocyst]] wall, but may be confused with other objects. Under UV [[fluorescence microscopy]], the [[oocyst]] wall autofluoresces. | ||
*An intense blue fluorescence is obtained with the preferred UV excitation filter set (330 to 365 nm). | *An intense blue [[fluorescence]] is obtained with the preferred UV excitation filter set (330 to 365 nm). | ||
*If this filter set is not available, a less intense green fluorescence can be obtained with blue excitation (450 to 490 nm). | *If this filter set is not available, a less intense green fluorescence can be obtained with blue excitation (450 to 490 nm). | ||
*A fluorescence microscope is required and this procedure does not provide a stained slide that can be archived. | *A [[fluorescence microscope]] is required and this procedure does not provide a stained slide that can be archived. | ||
*Both DIC and UV fluorescence microscopy are efficient and reliable approaches for identification of cyclospora. | *Both DIC and UV [[fluorescence microscopy]] are efficient and reliable approaches for identification of [[cyclospora]]. | ||
Shown below are images of oocysts of cyclospora in an unstained wet mount. | Shown below are images of oocysts of cyclospora in an unstained wet mount. | ||
| Line 29: | Line 29: | ||
====Modified Acid Fast Stain==== | ====Modified Acid Fast Stain==== | ||
*A blue-green background, or contrasting counterstain, of fecal debris allows the oocysts to stand out. | *A blue-green background, or contrasting counterstain, of fecal debris allows the oocysts to stand out. | ||
*The oocysts are variably stained: some will stain light pink to deep purple, while others may be unstained. | *The [[oocysts]] are variably stained: some will stain light pink to deep purple, while others may be unstained. | ||
*The oocysts | *The [[oocysts]] measure 8 to 10 μm and may not be perfectly round; some may appear collapsed or distorted on one side. | ||
*They may contain granules and/or have a wrinkled oocyst wall appearance (characteristics that distinguish oocysts from acid-fast artifacts). | *They may contain granules and/or have a wrinkled [[oocyst]] wall appearance (characteristics that distinguish oocysts from [[acid-fast]] artifacts). | ||
*This staining method is the easiest and most practical, and provides a stained slide that can be archived. | *This staining method is the easiest and most practical, and provides a stained slide that can be archived. | ||
*The variability in staining and confusion with artifacts is a cause of misdiagnosing this infection. | *The variability in [[staining]] and confusion with artifacts is a cause of misdiagnosing this infection. | ||
Shown below are images of oocysts of cyclospora stained with modified acid fast stain. | Shown below are images of oocysts of cyclospora stained with modified acid fast stain. | ||
| Line 43: | Line 43: | ||
====Safranin Stain==== | ====Safranin Stain==== | ||
*In the safranin stain, cylcospora | *In the safranin stain, cylcospora [[oocysts]] stain uniformly, red to reddish-orange. | ||
*This uniform staining decreases the risk of misdiagnosis. | *This uniform staining decreases the risk of misdiagnosis. | ||
*This technique requires heating, therefore additional equipment is necessary. | *This technique requires heating, therefore additional equipment is necessary. | ||
| Line 56: | Line 56: | ||
====Trichrome Stain==== | ====Trichrome Stain==== | ||
*[[Oocyst]]s may be detected, but should not be confirmed, by this method. | *[[Oocyst]]s may be detected, but should not be confirmed, by this method. | ||
*Trichrome stain is the routine staining technique for stool specimens in most laboratories and laboratorians should be familiar with the appearance of [[cyclospora]] stained with trichrome in order to detect oocysts during routine examinations. | *Trichrome stain is the routine staining technique for stool specimens in most laboratories and laboratorians should be familiar with the appearance of [[cyclospora]] stained with trichrome in order to detect [[oocysts]] during routine examinations. | ||
*This staining method is inadequate for definitive diagnosis because all oocysts will appear unstained. | *This staining method is inadequate for definitive diagnosis because all [[oocysts]] will appear unstained. | ||
*Oocysts appear as clear, round, wrinkled spheres, that measure between 8 to 10 μm. | *Oocysts appear as clear, round, wrinkled spheres, that measure between 8 to 10 μm. | ||
| Line 66: | Line 66: | ||
===Polymerase Chain Reaction=== | ===Polymerase Chain Reaction=== | ||
*Molecular diagnostic methods, such as polymerase chain reaction (PCR) analysis, are used to look for the parasite's DNA in the stool.<ref>http://www.cdc.gov/parasites/cyclosporiasis/diagnosis.html</ref> | *Molecular diagnostic methods, such as [[polymerase chain reaction]] ([[PCR]]) analysis, are used to look for the parasite's [[DNA]] in the [[stool]].<ref>http://www.cdc.gov/parasites/cyclosporiasis/diagnosis.html</ref> | ||
*Nested polymerase chain reaction (nested-PCR) assay targeting the 18S rRNA gene is the method of choice for | *Nested polymerase chain reaction (nested-PCR) assay targeting the 18S [[rRNA]] gene is the method of choice for [[cyclospora]] species identification. | ||
*Shown below is a cyclospora positive fecal specimen. The red arrow shows the diagnostic band for cyclospora cayetanensis (size: 308 bp). | *Shown below is a [[cyclospora]] positive fecal specimen. The red arrow shows the diagnostic band for [[cyclospora cayetanensis]] (size: 308 bp). | ||
{| | {| | ||
| Line 80: | Line 80: | ||
[[Category:Disease]] | [[Category:Disease]] | ||
[[Category:Infectious disease]] | [[Category:Infectious disease]] | ||
Revision as of 15:57, 18 September 2014
|
Cyclosporiasis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Cyclosporiasis laboratory findings On the Web |
|
American Roentgen Ray Society Images of Cyclosporiasis laboratory findings |
|
Risk calculators and risk factors for Cyclosporiasis laboratory findings |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Alejandro Lemor, M.D. [2]
Overview
The diagnosis of cyclospora infection is confirmed examining stool specimens and several stool samples are require for a more precise identification of the oocysts. Laboratory techniques used to detect the cyclospora oocytes in stool include acid-fast staining, ultraviolet (UV) fluorescence microscope, and polymerase chain reaction (PCR) analysis.[1]
Laboratory Findings
- Cyclospora infection is diagnosed by examining stool specimens.
- Laboratory testing for cyclospora is not routinely done in most U.S. laboratories, even when stool is tested for parasites. Therefore, if indicated, health care providers should specifically request testing for cyclospora.
- Laboratory diagnosis can be difficult as the cyclospora oocysts may not be detected in stool, even if the patient is symptomatic.
- Therefore, patients might need to submit several specimens collected on different days.
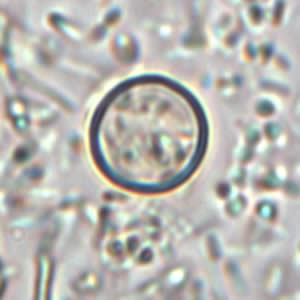
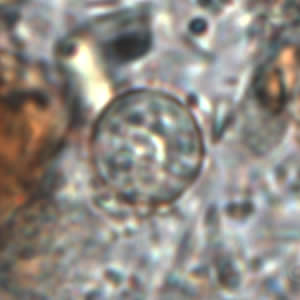
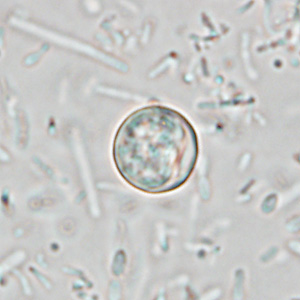
Stool Examination
Wet Mount
- In bright-field microscopy using differential interference contrast (DIC), oocysts appear as refractile spheres (8 to 10 μm) with a distinct oocyst wall, but may be confused with other objects. Under UV fluorescence microscopy, the oocyst wall autofluoresces.
- An intense blue fluorescence is obtained with the preferred UV excitation filter set (330 to 365 nm).
- If this filter set is not available, a less intense green fluorescence can be obtained with blue excitation (450 to 490 nm).
- A fluorescence microscope is required and this procedure does not provide a stained slide that can be archived.
- Both DIC and UV fluorescence microscopy are efficient and reliable approaches for identification of cyclospora.
Shown below are images of oocysts of cyclospora in an unstained wet mount.
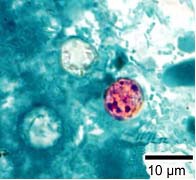
Modified Acid Fast Stain
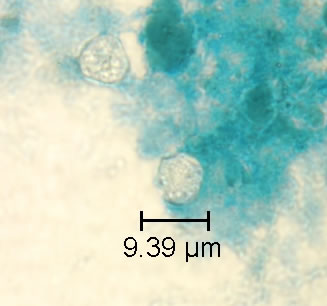
- A blue-green background, or contrasting counterstain, of fecal debris allows the oocysts to stand out.
- The oocysts are variably stained: some will stain light pink to deep purple, while others may be unstained.
- The oocysts measure 8 to 10 μm and may not be perfectly round; some may appear collapsed or distorted on one side.
- They may contain granules and/or have a wrinkled oocyst wall appearance (characteristics that distinguish oocysts from acid-fast artifacts).
- This staining method is the easiest and most practical, and provides a stained slide that can be archived.
- The variability in staining and confusion with artifacts is a cause of misdiagnosing this infection.
Shown below are images of oocysts of cyclospora stained with modified acid fast stain.
 |
 |
Safranin Stain
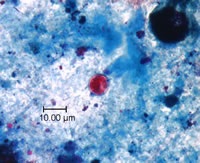
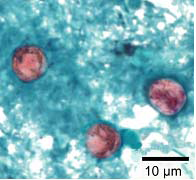
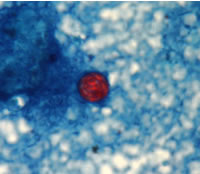
- In the safranin stain, cylcospora oocysts stain uniformly, red to reddish-orange.
- This uniform staining decreases the risk of misdiagnosis.
- This technique requires heating, therefore additional equipment is necessary.
Shown below are images of oocysts of cyclospora stained with safranin stain.
 |
 |
 |
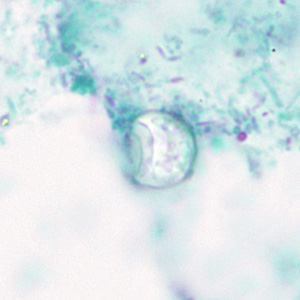
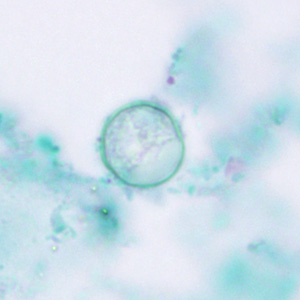
Trichrome Stain
- Oocysts may be detected, but should not be confirmed, by this method.
- Trichrome stain is the routine staining technique for stool specimens in most laboratories and laboratorians should be familiar with the appearance of cyclospora stained with trichrome in order to detect oocysts during routine examinations.
- This staining method is inadequate for definitive diagnosis because all oocysts will appear unstained.
- Oocysts appear as clear, round, wrinkled spheres, that measure between 8 to 10 μm.
 |
 |
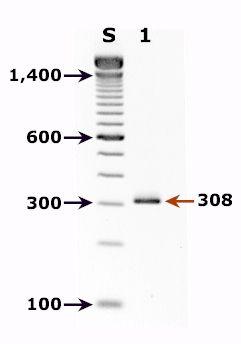
Polymerase Chain Reaction
- Molecular diagnostic methods, such as polymerase chain reaction (PCR) analysis, are used to look for the parasite's DNA in the stool.[3]
- Nested polymerase chain reaction (nested-PCR) assay targeting the 18S rRNA gene is the method of choice for cyclospora species identification.
- Shown below is a cyclospora positive fecal specimen. The red arrow shows the diagnostic band for cyclospora cayetanensis (size: 308 bp).
 |