Coronary artery bypass surgery perioperative medical therapy
|
Coronary Artery Bypass Surgery Microchapters | |
|
Pathophysiology | |
|---|---|
|
Diagnosis | |
|
Treatment | |
|
Perioperative Monitoring | |
|
Surgical Procedure | |
|
Special Scenarios | |
|
Coronary artery bypass surgery perioperative medical therapy On the Web | |
|
FDA on Coronary artery bypass surgery perioperative medical therapy | |
|
CDC on Coronary artery bypass surgery perioperative medical therapy | |
|
Coronary artery bypass surgery perioperative medical therapy in the news | |
|
Blogs on Coronary artery bypass surgery perioperative medical therapy|- |
|
|
Directions to Hospitals Performing Coronary artery bypass surgery perioperative medical therapy | |
|
Risk calculators for Coronary artery bypass surgery perioperative medical therapy | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editors-in-Chief: Cafer Zorkun, M.D., Ph.D. [2],Mohammed A. Sbeih, M.D. [3]
Perioperative medical therapy
Aspirin
Aspirin is a simple cost effective therapy that has been associated with improved clinical outcomes among patients undergoing CABG. The optimal timing of aspirin administration appears to be in the 48 hours immediately after CABG.[1][2][3][4][5][6][7][8] [9][10][11][12][13][14]
Rigorous randomized trials have been required to document the benefits of aspirin, to determine the optimal timing of aspirin, and to overcome fears surrounding the risk of bleeding associated with administration of aspirin in the setting of CABG. There has been a hesitancy to recommend the administration of antiplatelet agents in the setting of CABG for several reasons:
- Platelet counts and platelet concentration are reduced during the peri-operative period as a result of sequestration and hemodilution[15]
- Platelet function is impaired following CABG due to both hypothermia [16] and/or mechanical filtering.[17][18][19]
In so far as the focus of clinical care has at times been on reducing the risk of bleeding rather than on reducing the risk of thrombosis, there has likewise been a tendency to discontinue aspirin and reverse anticoagulant therapy before surgery, to administer platelet transfusions during surgery,[20][21][22] There is little randomized controlled data to support these practices adn non-randomized data suggest that these practices are associated with a significant increase in death and ischemic events.[6]
Benefit of early post-operative aspirin administration
In one of the first studies in this field, Goldman et al compared the rate of SVG patency among CABG patients treated with a variety of antiplatelet regimens.[1] All therapies except aspirin were started 48 hours before CABG. When aspirin was part of the regime, one 325 mg dose was given 12 hours pre-operatively, and the assigned therapy was maintained thereafter. The 60 day rates of angiographic patency (555 patients with 1,781 grafts) were:
- Aspirin, 325 mg daily: 93.5%
- Aspirin, 325 mg three times daily: 92.3%
- Aspirin plus dipyridamole (325 mg and 75 mg, respectively, three times daily): 91.9%
- Sulfinpyrazone (267 mg three times daily): 90.2%
- Placebo (three times daily): 85.2%
(P<0.05 for all aspirin regimens vs placebo)
Aspirin was associated with a greater median chest tube drainage within the first 35 hours post-operatively compared with placebo (p<0.02):
- Aspirin daily (965 ml)
- Aspirin three times daily (1175 ml)
- Aspirin plus dipyridamole (1000 ml)
- Sulfinpyrazone (775 ml)
- Placebo (805 ml)
The rate of reoperation was higher (p<0.01) among patients treated with aspirin (6.5%) than among those patients not treated with aspirin (1.7%).
At one year of follow-up in the same cohort of patients (n=406 patients with 1,315 SVGs), the rate of SVG occlusion was 15.8% in all the aspirin groups combined vs 22.6% among those treated with placebo (p = 0.029).[2] This benefit was signficant among those SVGs in which the target vessel was less than or equal to 2.0 mm in diameter (20.1% vs 32.3% for the placebo group (p = 0.008), while in those SVGs anastomosed to target vessels > 2.0 mm in diameter there was no difference in the rate of SVG occlusion (8.7% vs. 9.0%, p = 0.918).
While there were benefits in early patency and patency at one year in this cohort of patients, between years one and three, there was no benefit in the rate of occlusion. Among those SVGs that were patent at 1 year, the occlusion rate at 3 years was 4.8% for aspirin treated patients vs 4.2% for placebo treated patients (p=NS).[3]
The benefits of early antiplatelet therapy were also documented by Chesebro et al who performed a double blind randomized trial evaluating the benefit of dipyridamole (administered two days before operation) plus aspirin (added seven hours after operation) in 407 patients.[4] At one month, the angiographic rate of SVG occlusion on a per lesion basis was 3% vs 10% of grafts, and on a per patient basis the rate of having at least one SVG occluded was 8% vs 21% for treated vs untreated patients respectively. Likewise, at one year, the angiographic rate of SVG occlusion on a per lesion basis was 11% vs 25% of grafts, and on a per patient basis the rate of having at least one SVG occluded was 22% vs 47% for treated vs untreated patients respectively.[5]
In a non-randomized retrospective analysis of 7,500 variables, Mangano et al evaluated the relationship between early aspirin use and clinical outcomes in 5,065 patients at 70 centers in 17 countries.[6] Mortality was 1.3% among those patients treated with aspirin in the first 48 hours after CABG vs 4.0% among those who were not treated with aspirin (p<0.001). Likewise, aspirin therapy reduced the risk of MI from 5.4% to 2.8% (p<0.001), the risk of stroke from 2.6% to 1.3% (p=0.01), the risk of bowel infarction from 0.8% to 0.3% (p=0.01) and the risk of renal failure from 3.4% to 0.9%, p<0.001). Aspirin treatment was not associated with an increased risk of hemorrhage or impaired wound healing.
Pre-Operative vs Post-Operative Administration of Aspirin
Although the aforementioned studies demonstrated improved early and late patency with the pre-opeartive administration of aspirin, there was a higher rate of bleeding. Goldman et al conduted a prospective, randomized, double-blind, placebo-controlled trial to compare the safety and effectiveness of 325 mg of aspirin therapy initiated either the night before before CABG vs aspirin initiated via nasogastric tube 6 hours post-operatively.[23] The rate of saphenous vein graft occlusion rate was 7.4% vs 7.8% for pre vs post-operative aspirin administration. Pre-operative aspirin was associated with a greater amount of blood volume transfused (900 versus 725 cc, p = 0.006), greater chest tube drainage at 6 hours (500 vs 448 cc, p=0.011) and a higher rate of re-operation for bleeding (6.3% vs 2.4%, p = 0.036).
Post-operative clopidogrel
There are no randomized controlled trials that demonstrate a benefit of clopidogrel in the post-operative management of CABG patients.[24] Furthermore, retrospective subgroup analyses from large trials of acute coronary syndrome patients (1 trial) and stable coronary artery disease patients (3 trials) have not demonstrated a benefit of post-operative clopidogrel. They did, however, demonstrate a trend toward an increased risk of major and minor bleeding with the combined use of clopidogrel plus aspirin.
Percutaneous coronary intervention (PCI) to treat saphenous vein graft failure
There are many different choices to consider when deciding the most appropriate treatment for SVG stenosis, including PTCA, PCI with bare metal or drug-eluting stents, PCI with covered stents, embolic protection devices, debulking/thrombus removal, and surgical revascularization.
Percutaneous transluminal coronary angioplasty (PTCA)
PTCA has high initial revascularization success rates in the treatment of SVG stenosis. However, it is also associated with high rates of periprocedural complications, including acute vessel closure secondary to dissection and in-situ thrombosis. Additional complications include distal embolization and no reflow, which can lead to periprocedural infarction.
In modern interventional cardiology, PTCA is not often used as the sole means of treatment for SVG stenosis. Instead, stenting has become the cornerstone of treatment, while the use of PTCA has been limited to pre-dilation and post-dilation.
PCI with bare metal stents (BMS) or drug-eluting stents (DES)
Most current vein graft treatment strategies utilize PCI with stents (BMS or DES), since stenting is a superior treatment when compared to PTCA alone. As demonstrated in the SAVED (Saphenous Vein De Novo) Trial[25], the use of stents is associated with higher revascularization success rates, decreased restenosis rates, and improved clinical outcomes when compared to PTCA. Generally, DES are preferred over BMS, since DES are associated with reduced rates of restenosis and target vessel revascularization.
Despite their higher success rates, stents are not immune to restenosis. Predictors for restenosis include long stent length, multiple stents, overlapping stents, smaller vessel size, diabetes mellitus, and stenosis at the coronary or aortic anastomosis.
PCI with covered stents
Theoretically, stents covered with a polymer membrane would have higher success rates than standard BMS and DES. One would expect covered stents to effectively trap friable atheroma and isolate the graft lumen from the diseased wall, thereby reducing incidence of restenosis, distal embolization, and no reflow in comparison to traditional stents. However, the RECOVERS (The Randomized Evaluation of polytetrafluoroethylene COVERed stent in Saphenous vein grafts)[26] and STING (STents IN Grafts)[27] trials did not show any advantage in using covered stents when compared to bare metal stents for SVG lesions.
Embolic protection devices
During PCI of SVGs, atheroembolic debris can be liberated. This debris contains vasoactive substances that can contribute to no reflow, which can in turn considerably increase the risk of major adverse clinical events (MACE)[28]. Fortunately, embolic protection devices help capture this debris and improve outcomes in PCI for SVG stenosis. Therefore, it is recommended that these devices should be utilized in the intervention of most SVG lesions.
Currently, the FDA has approved five embolic protection devices in the United States. Specifically, these devices include one distal occlusion device, three filters, and one proximal occlusion device.
The FDA-approved distal occlusion device is called the PercuSurge Guardwire®, which involves inflating a balloon distal to the stenosis to occlude flow, thereby trapping the debris and vasoactive substances and preventing them from flowing downstream. Due to its small size, it requires little landing zone to deploy. The SAFER (Saphenous vein graft Angioplasty Free of Emboli Randomized) trial[29] showed that when compared to conventional guidewires, balloon occlusion devices (PercuSurge Guardwire®) reduced the rates of infarction and no-reflow after intervention. Despite these advantages, the PercuSurge Guardwire® may not be the best option for all, as some patients may not tolerate the necessary 3-5 minutes of ischemic time associated with this device. Additionally, it is known to cause both hemodynamic and arrhythmic complications.
Filter devices allow continual distal perfusion while macroscopic emboli are trapped in the filter. The FIRE (FilterWire EX During Transluminal Intervention of Saphenous Vein Grafts) trial[30][31] showed that FilterWire may be preferred over PercuSurge Guardwire® due to improved clinical outcomes. While they may reduce ischemic time, filter devices are associated with their own set of potential complications. They are more difficult to deliver than balloon occlusion devices, so their own delivery may lead to distal embolization, and they may not trap microscopic mediators of no reflow. Additionally, they require a significant landing zone distal to the lesion for the filter placement, which can be problematic for certain distal lesions that do not have enough room. There have also been case reports of filter entrapment in the graft after the completion of the PCI.
The FDA-approved proximal occlusion device is called the Proxis® device. Some advantages of this decide are that its deployment does not require crossing the stenosis, it provides superior support that is helpful where balloon or stent delivery is difficult, and it provides protected crossing of the lesion, if required. However, as shown by the PROXIMAL (Proximal Protection During Saphenous Vein Graft Intervention Using the Proxis Embolic Protection System) trial[32], in terms of overall outcomes, there is no significant difference in death, MI, or target vessel revascularization (TVR) between distal and proximal embolic protection devices.
Debulking/thrombus removal
Data has not demonstrated a durable clinical benefit associated with debulking/thrombus removal. However, there are certain situations in which debulking techniques may be useful when treating saphenous vein grafts. For instance, severely calcified and stenotic lesions can make regular stent insertion especially difficult. When SVG lesions are too calcified to be crossed by a balloon or adequately dilated prior to stent placement, debulking and thrombus removal can change the compliance of the vessel wall. In addition, this technique is also useful if a lesion is at the aorto-ostial junction. Adjunctive stenting leads to better short and long term results.
There are several debulking/thrombus removal techniques, including directional coronary atherectomy, transluminal extraction catheter thrombectomy, rotational atherectomy, and laser atherectomy.
- Directional coronary atherectomy (DCA) uses a circular cutting blade that excises atheroma into a chamber for removal. It is useful for aorto-ostial lesions and focal lesions in large vessels. However, due to its bulky nature, it is generally not used in vessels with angulation, tortuosity, or heavy calcification. CAVEAT II (Coronary Angioplasty Versus Excisional Atherectomy Trial)[33] examined how PTCA and DCA compared in the treatment of patients with coronary artery bypass graft stenoses. This study demonstrated that DCA was associated with higher initial angiographic success rates and larger acute luminal dimensions in comparison to PTCA. However, despite these successes, DCA also displayed an increased rate of non-Q wave myocardial infusion and distal embolization than PTCA. Furthermore, both techniques displayed similar restenosis rates.
- Additionally, a retrospective study compared DCA vs. PTCA alone vs. PCI with stenting in SVG lesions. It showed no differences in mortality, angina, infarction, or repeat revascularization among the different methods. However, this study displayed increased angiographic complications with DCA use.
- Transluminal extraction catheter (TEC) thrombectomy is designed to remove thrombus from SVGs prior to stenting. It operates through the use of cutting blades with a rotating catheter and an external suction device. However, because the TEC Best trial showed no benefit of TEC prior to the stenting of SVGs, this technique has fallen out of favor. Furthermore, TEC is also associated with a significant incidence of distal embolization and no reflow.
- Rotational atherectomy (RA) uses a rotating cutting blade to grind calcified atheroma. Despite its ability to grind calcification, this method is associated with high rates of no reflow, distal embolization, perforation, and dissection. Furthermore, this method is contraindicated for lesions located in the body of SVGs or in degenerated vein grafts.
- Laser atherectomy uses monochromatic light energy to disrupt plaques. Despite this approach's innovation, there is no evidence that this strategy improves outcomes in lesions, and it has been complicated by high rates of dissection and perforation.
Surgical revascularization
Given increased perioperative mortality, surgical revascularization is not an optimal treatment strategy, as many patients with graft disease are poor surgical candidates. However, surgery may be required in patients with multi-vessel disease and when PCI fails.
Additionally, reoperation is not strongly encouraged, as it does not provide the same level of revascularization and resolution of angina as the initial procedure. Furthermore, a LIMA may be jeopardized in a reoperation.
Making a selection
At the earliest signs of recurrent ischemia, it is important to strongly consider the possibility of a patent but stenosed SVG, so that the graft lesion can be treated before the graft becomes completely occluded. Prompt treatment is essential, since a graft is lost once it becomes completely occluded.
Regardless of treatment choice, all patients should be given statins and aspirin (begun immediately following CABG), which are effective in the secondary prevention of SVG stenosis.
For most SVG lesions, PCI with stenting appears to be the therapy of choice. DES are associated with a decreased restenosis rate over BMS, and should be used preferentially if the patient is able to tolerate dual platelet therapy for a minimum of a year. Furthermore, embolic protection devices should be strongly considered for all SVG lesions, especially those with high risks for distal embolization. In cases in which stent delivery and expansion may be difficult due to heavily calcified and stenotic lesions, atherectomy devices, used with stenting, may be considered. Furthermore, these devices can be useful in lesions that are aorto-ostial.
Zoghbi et al. conducted a study to investigate the role of pretreatment with nitroprusside before SVG intervention[34]. They studied sixty-four consecutive patients with normal preprocedural cardiac enzymes that underwent SVG PCI, without the use of embolic protection devices. They found that pretreatment with nitroprusside results in a lower magnitude and frequency of post-procedural cardiac enzyme elevation. Thus, it is important to consider nitroprusside use.
Finally, while GP IIb/IIIa inhibitors are frequently used in the setting of SVG intervention, their benefit has not been fully evaluated in randomized trials of this lesion subset.
Other concerns
As with all medical procedures, complications for SVG intervention can occur. Risk factors for complications include: older graft age (>3-5 years), the presence of thrombus, and diffuse disease.
Although PCI with stenting is effective for focal lesions, there is uncertainty regarding the best treatment for diffusely degenerated SVGs. In these cases, it is often a better choice to abandon the graft and intervene on the native vessel instead.
As mentioned above, prevention of no reflow should be attempted with embolic protection devices, pretreatment using nitroprusside and the avoidance of high-pressure inflations and unnecessary pre/post-dilation and oversizing. However, in the event that no reflow develops, it should be aggressively managed with intracoronary vasodilators (i.e. diltiazem, nicardipine, adenosine, and nitroprusside).
Pathological findings
-
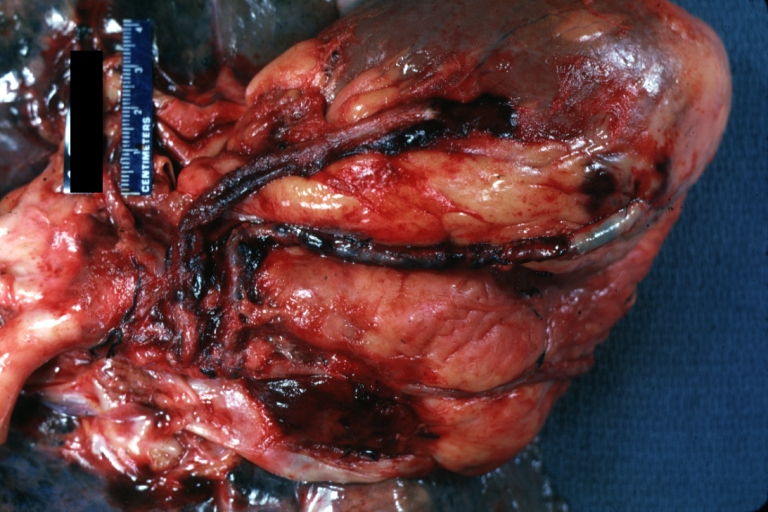
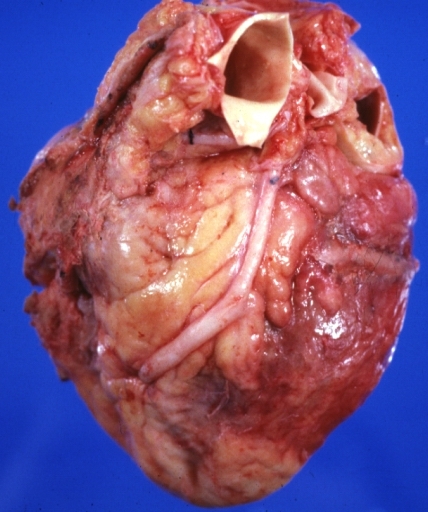
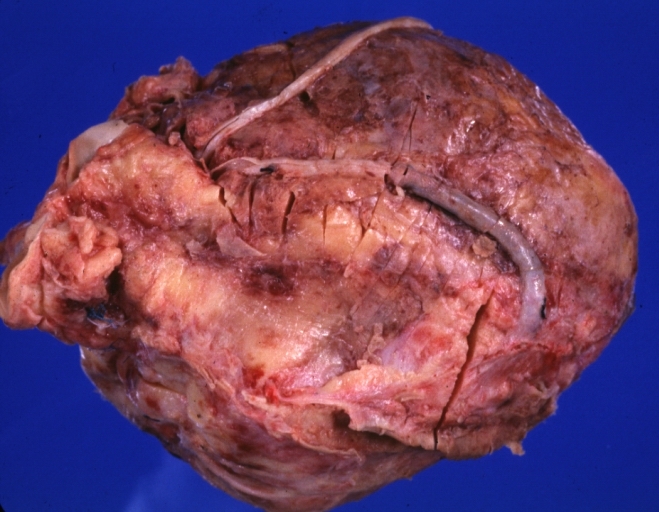
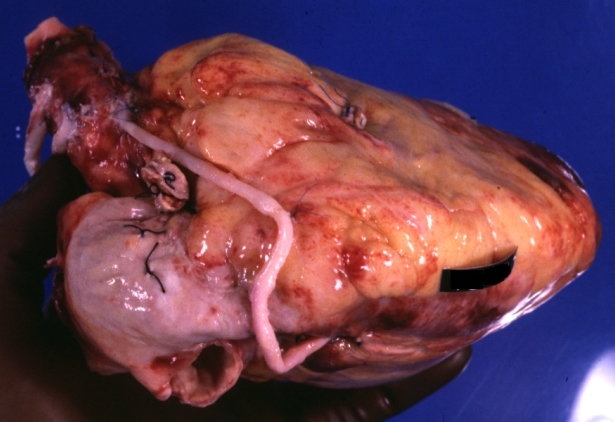
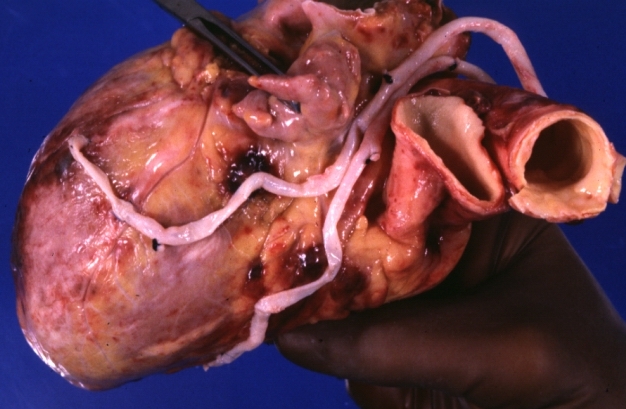
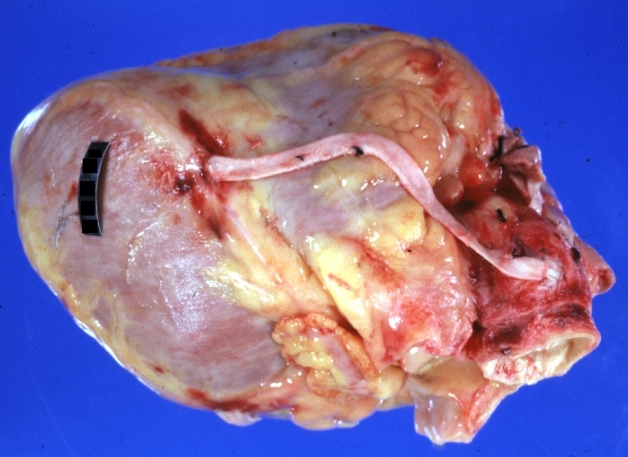
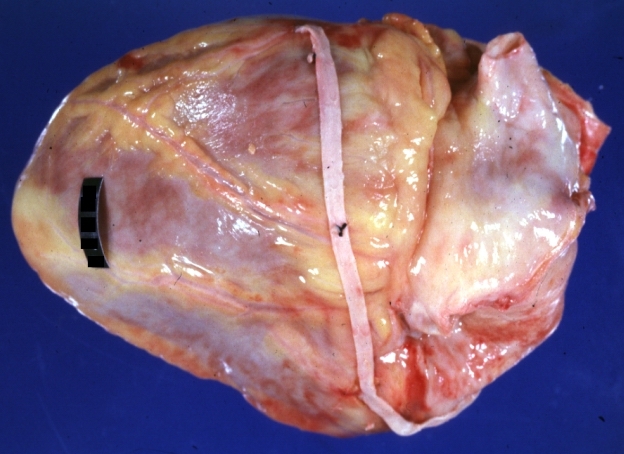
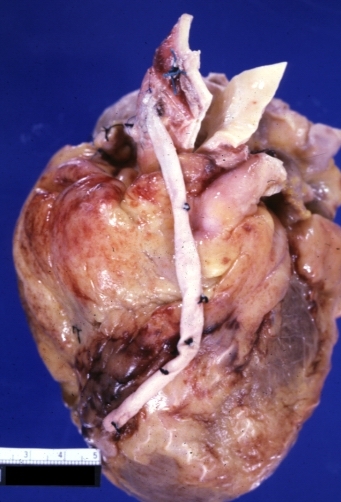
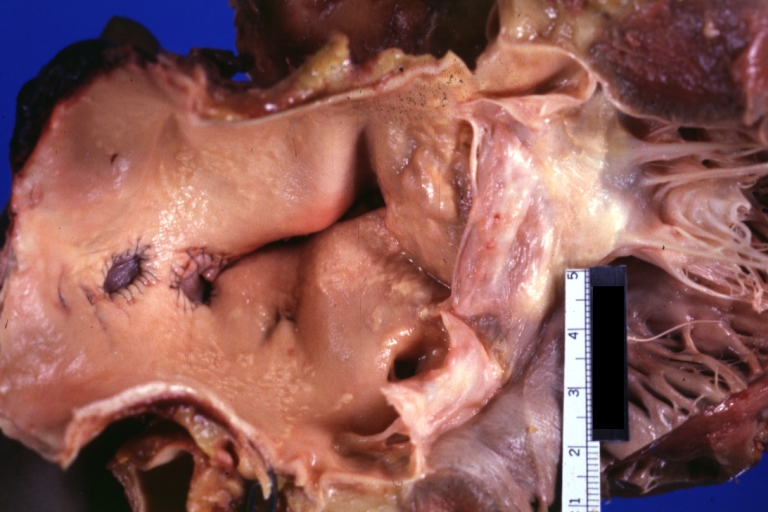
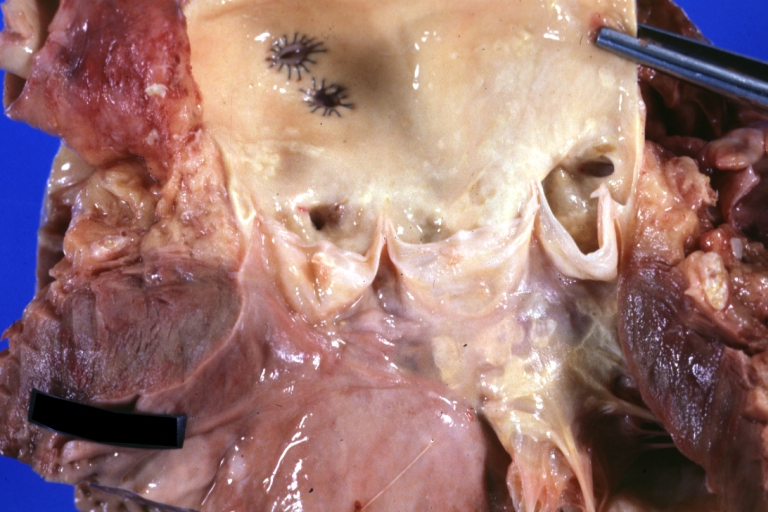
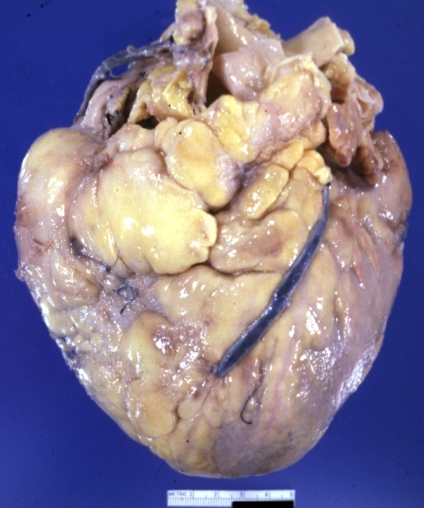
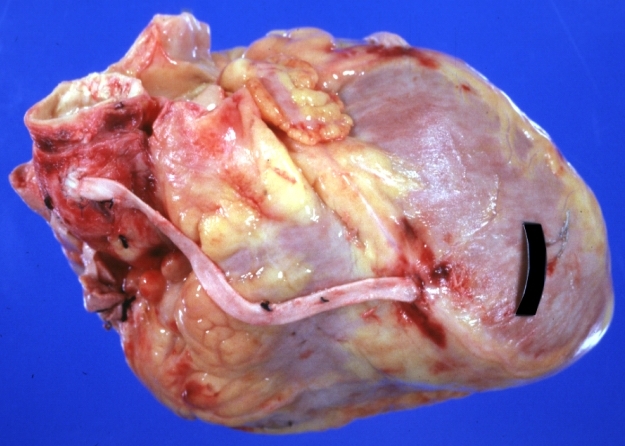
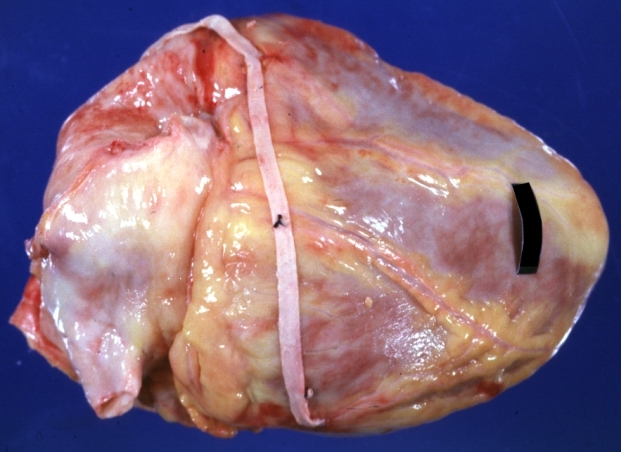
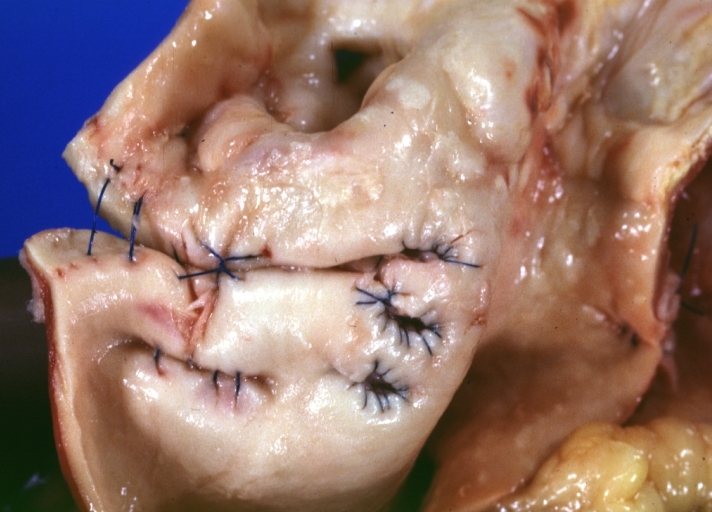
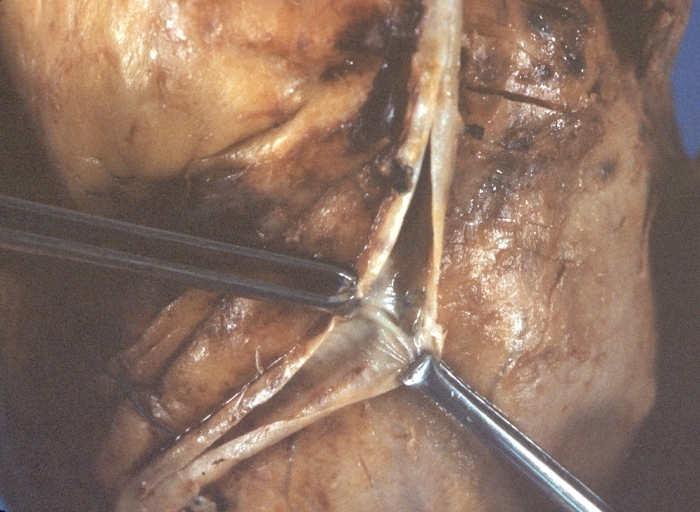
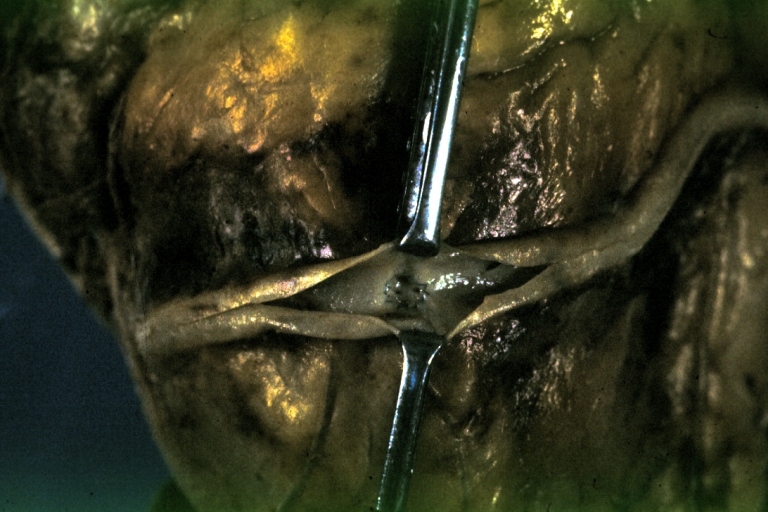
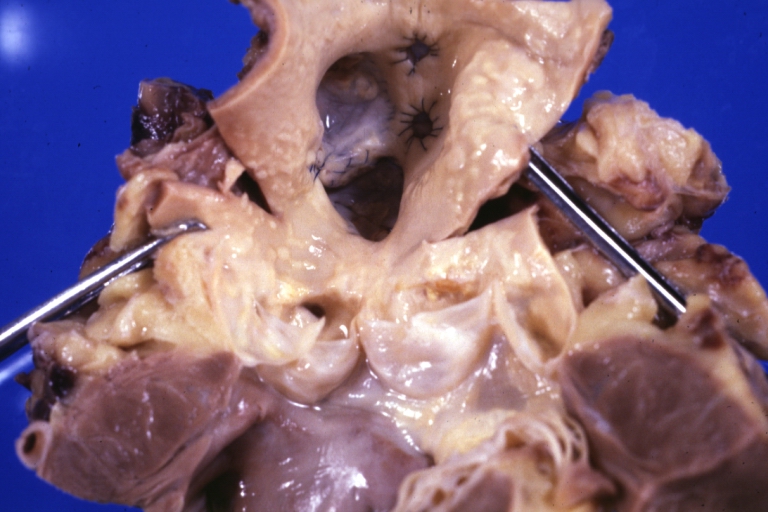
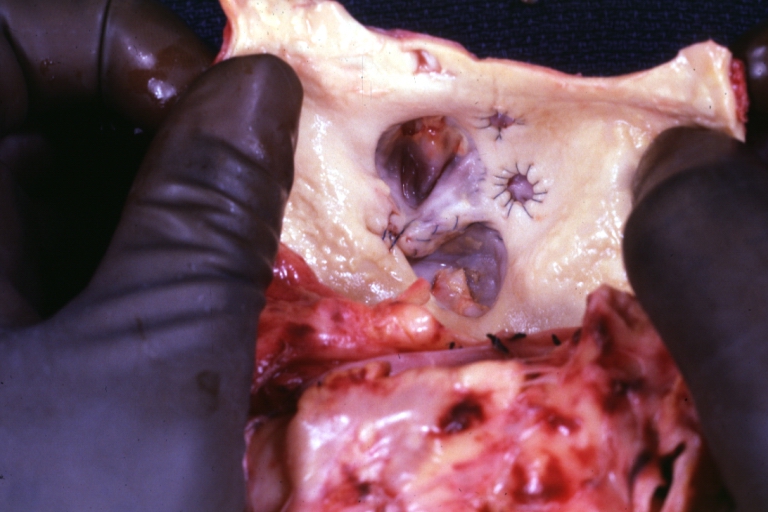
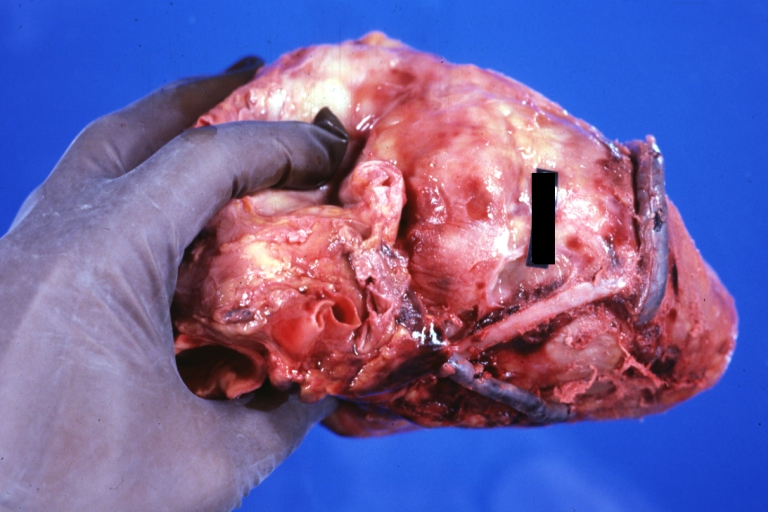
Saphenous vein coronary bypass graft: Gross, natural color, external view of heart with thrombosed veins
-
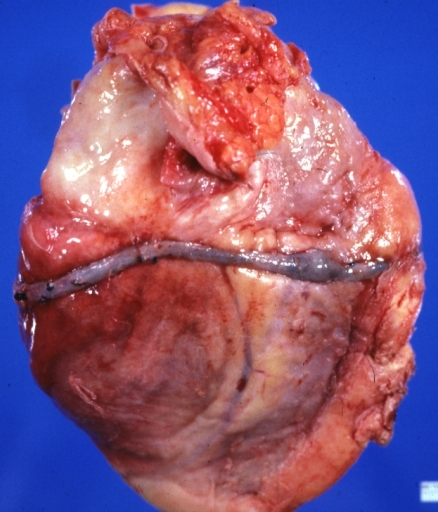
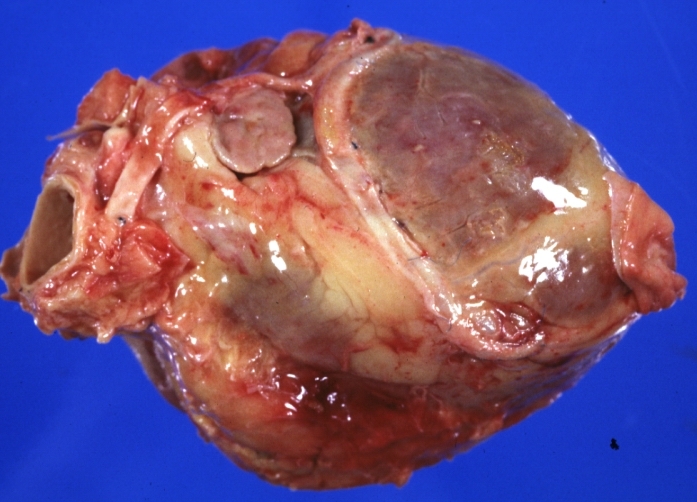
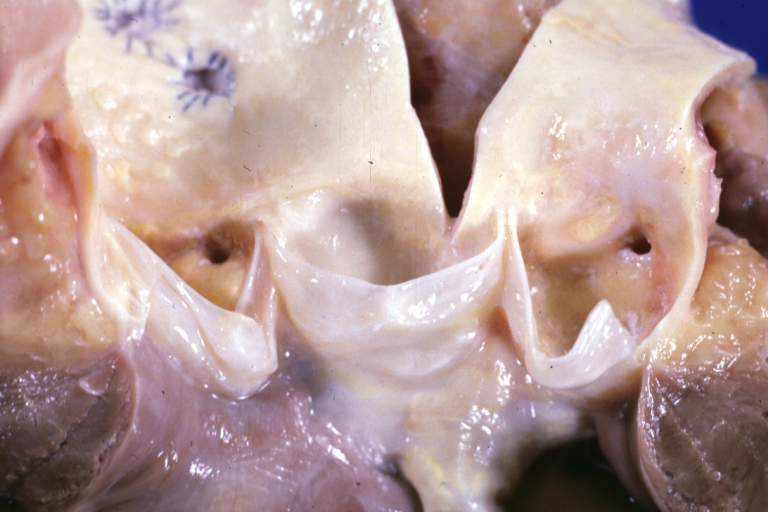
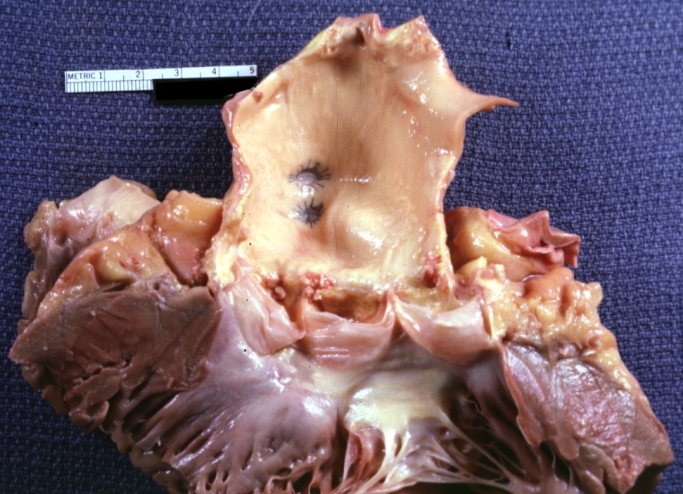
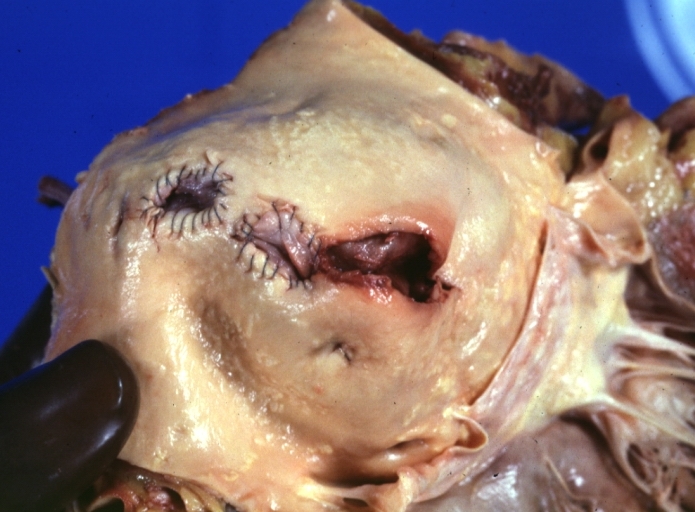
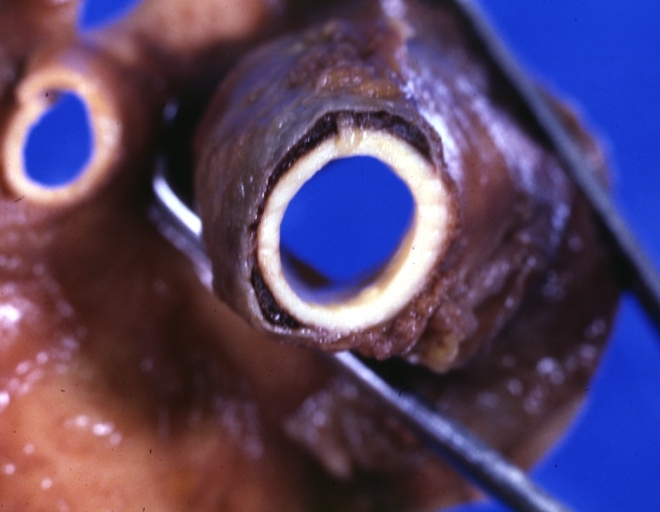
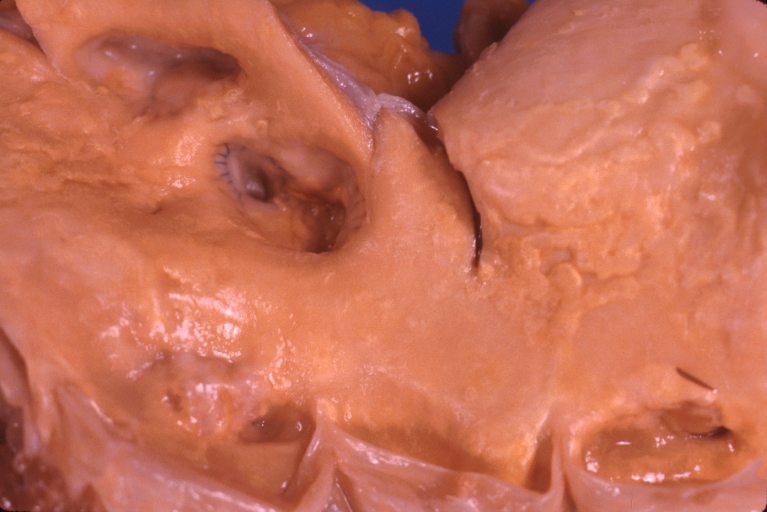
Saphenous vein coronary bypass graft: Thrombosis, Acute: Gross, fixed tissue but well shown cross sections of bypass graft and anastomotic site with thrombosis. 61 yo male, with and acute infarct treated with streptokinase and two days later had bypass. Died 5 days post op. Two veins are thrombosed
-
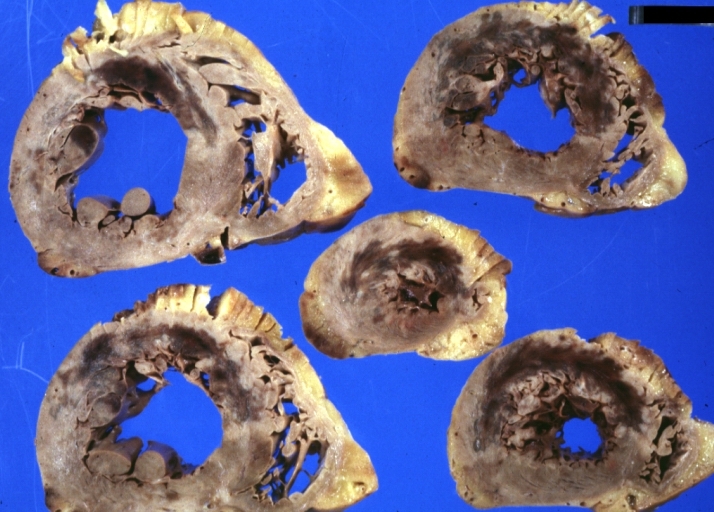
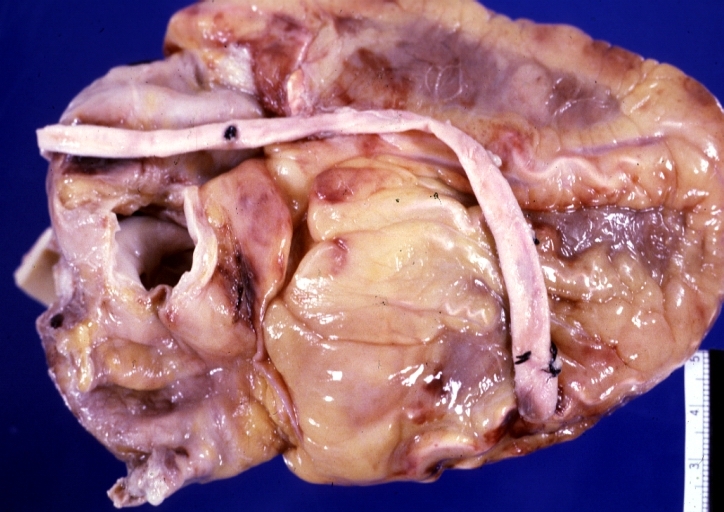
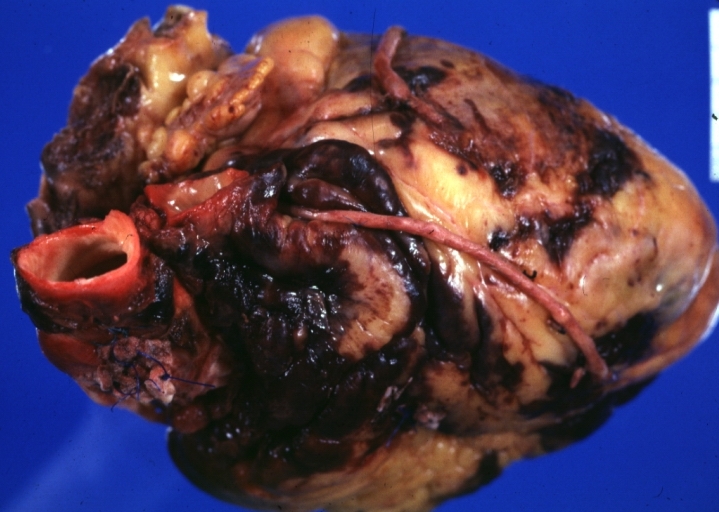
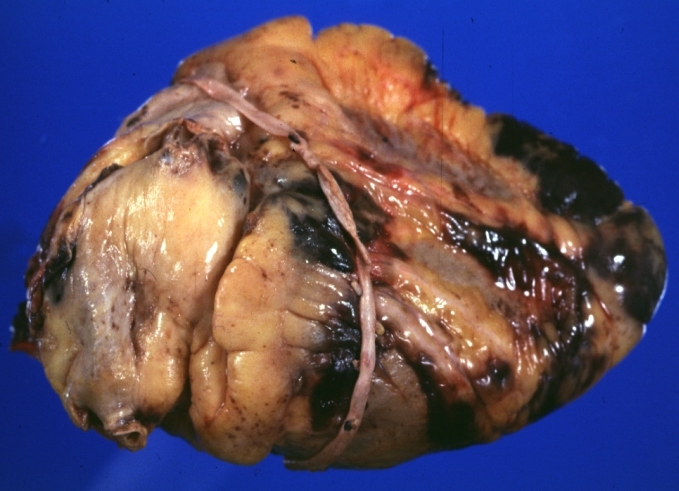
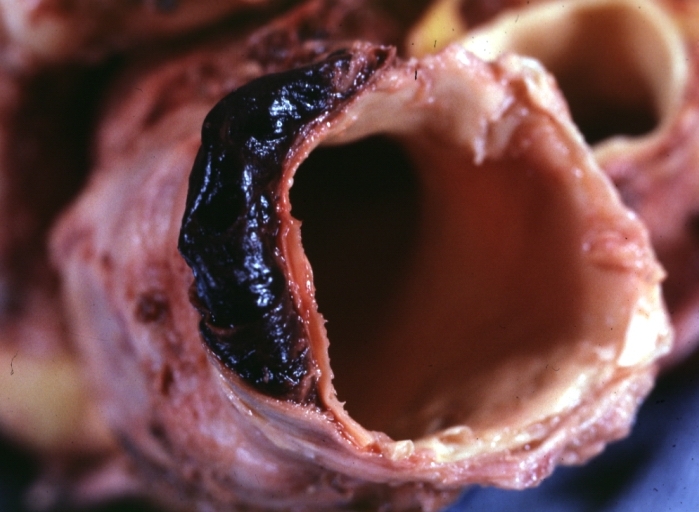
Myocardial Infarct Acute Reflow Type: Gross, fixed tissue but good color. A very enlarged heart with moderate LV dilation and high anterior wall hemorrhagic infarct. Initially treated with streptokinase and two days later had saphenous vein grafts. Both grafts are thrombosed. He died after 5 days
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
References
- ↑ 1.0 1.1 Goldman S, Copeland J, Moritz T, Henderson W, Zadina K, Ovitt T, Doherty J, Read R, Chesler E, Sako Y (1988). "Improvement in early saphenous vein graft patency after coronary artery bypass surgery with antiplatelet therapy: results of a Veterans Administration Cooperative Study". Circulation. 77 (6): 1324–32. PMID 3286040. Retrieved 2010-07-22. Unknown parameter
|month=ignored (help) - ↑ 2.0 2.1 Goldman S, Copeland J, Moritz T, Henderson W, Zadina K, Ovitt T, Doherty J, Read R, Chesler E, Sako Y (1989). "Saphenous vein graft patency 1 year after coronary artery bypass surgery and effects of antiplatelet therapy. Results of a Veterans Administration Cooperative Study". Circulation. 80 (5): 1190–7. PMID 2680158. Retrieved 2010-07-22. Unknown parameter
|month=ignored (help) - ↑ 3.0 3.1 Goldman S, Copeland J, Moritz T, Henderson W, Zadina K, Ovitt T, Kern KB, Sethi G, Sharma GV, Khuri S (1994). "Long-term graft patency (3 years) after coronary artery surgery. Effects of aspirin: results of a VA Cooperative study". Circulation. 89 (3): 1138–43. PMID 8124800. Retrieved 2010-07-22. Unknown parameter
|month=ignored (help) - ↑ 4.0 4.1 Chesebro JH, Clements IP, Fuster V, Elveback LR, Smith HC, Bardsley WT, Frye RL, Holmes DR, Vlietstra RE, Pluth JR, Wallace RB, Puga FJ, Orszulak TA, Piehler JM, Schaff HV, Danielson GK (1982). "A platelet-inhibitor-drug trial in coronary-artery bypass operations: benefit of perioperative dipyridamole and aspirin therapy on early postoperative vein-graft patency". N. Engl. J. Med. 307 (2): 73–8. PMID 7045659. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ 5.0 5.1 Chesebro JH, Fuster V, Elveback LR, Clements IP, Smith HC, Holmes DR, Bardsley WT, Pluth JR, Wallace RB, Puga FJ (1984). "Effect of dipyridamole and aspirin on late vein-graft patency after coronary bypass operations". N. Engl. J. Med. 310 (4): 209–14. PMID 6361561. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ 6.0 6.1 6.2 Mangano DT (2002). "Aspirin and mortality from coronary bypass surgery". N. Engl. J. Med. 347 (17): 1309–17. doi:10.1056/NEJMoa020798. PMID 12397188. Retrieved 2010-07-22. Unknown parameter
|month=ignored (help) - ↑ Lorenz RL, Schacky CV, Weber M; et al. (1984). "Improved aortocoronary bypass patency by low-dose aspirin (100 mg daily). Effects on platelet aggregation and thromboxane formation". Lancet. 1 (8389): 1261–4. PMID 6144975. Unknown parameter
|month=ignored (help) - ↑ Hockings BE, Ireland MA, Gotch-Martin KF, Taylor RR (1993). "Placebo-controlled trial of enteric coated aspirin in coronary bypass graft patients. Effect on graft patency". Med. J. Aust. 159 (6): 376–8. PMID 8377686. Unknown parameter
|month=ignored (help) - ↑ Sanz G, Pajarón A, Alegría E; et al. (1990). "Prevention of early aortocoronary bypass occlusion by low-dose aspirin and dipyridamole. Grupo Español para el Seguimiento del Injerto Coronario (GESIC)". Circulation. 82 (3): 765–73. PMID 2203555. Unknown parameter
|month=ignored (help) - ↑ Gavaghan TP, Gebski V, Baron DW (1991). "Immediate postoperative aspirin improves vein graft patency early and late after coronary artery bypass graft surgery. A placebo-controlled, randomized study". Circulation. 83 (5): 1526–33. PMID 2022014. Unknown parameter
|month=ignored (help) - ↑ Sharma GV, Khuri SF, Josa M, Folland ED, Parisi AF (1983). "The effect of antiplatelet therapy on saphenous vein coronary artery bypass graft patency". Circulation. 68 (3 Pt 2): II218–21. PMID 6347428. Unknown parameter
|month=ignored (help) - ↑ Brown BG, Cukingnan RA, DeRouen T; et al. (1985). "Improved graft patency in patients treated with platelet-inhibiting therapy after coronary bypass surgery". Circulation. 72 (1): 138–46. PMID 3874009. Unknown parameter
|month=ignored (help) - ↑ McEnany MT, Salzman EW, Mundth ED; et al. (1982). "The effect of antithrombotic therapy on patency rates of saphenous vein coronary artery bypass grafts". J. Thorac. Cardiovasc. Surg. 83 (1): 81–9. PMID 7033673. Unknown parameter
|month=ignored (help) - ↑ Goldman S, Copeland J, Moritz T; et al. (1990). "Internal mammary artery and saphenous vein graft patency. Effects of aspirin". Circulation. 82 (5 Suppl): IV237–42. PMID 2225410. Unknown parameter
|month=ignored (help) - ↑ Khuri SF, Wolfe JA, Josa M, Axford TC, Szymanski I, Assousa S, Ragno G, Patel M, Silverman A, Park M (1992). "Hematologic changes during and after cardiopulmonary bypass and their relationship to the bleeding time and nonsurgical blood loss". J. Thorac. Cardiovasc. Surg. 104 (1): 94–107. PMID 1614220. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Hessel EA, Schmer G, Dillard DH (1980). "Platelet kinetics during deep hypothermia". J. Surg. Res. 28 (1): 23–34. PMID 7359906. Retrieved 2010-07-22. Unknown parameter
|month=ignored (help) - ↑ Kestin AS, Valeri CR, Khuri SF, Loscalzo J, Ellis PA, MacGregor H, Birjiniuk V, Ouimet H, Pasche B, Nelson MJ (1993). "The platelet function defect of cardiopulmonary bypass". Blood. 82 (1): 107–17. PMID 7686785. Retrieved 2010-07-22. Unknown parameter
|month=ignored (help) - ↑ Morse DS, Adams D, Magnani B (1998). "Platelet and neutrophil activation during cardiac surgical procedures: impact of cardiopulmonary bypass". Ann. Thorac. Surg. 65 (3): 691–5. PMID 9527196. Retrieved 2010-07-22. Unknown parameter
|month=ignored (help) - ↑ Rinder CS, Mathew JP, Rinder HM, Bonan J, Ault KA, Smith BR (1991). "Modulation of platelet surface adhesion receptors during cardiopulmonary bypass". Anesthesiology. 75 (4): 563–70. PMID 1718190. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Stover EP, Siegel LC, Parks R, et al. Variability in transfusion practice for coronary artery bypass surgery persists despite national consensus guidelines: a 24-institution study. Anesthesiology 1998;88:327-33.
- ↑ Spiess BD, Ley C, Body SC, et al. Hematocrit value on intensive care unit entry influences the frequency of Q-wave myocardial infarction after coronary artery bypass grafting. J Thorac Cardiovasc Surg 1998;116:460-7. and to administer prothrombotic agents(antifibrinolytic agents).
- ↑ Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA guidelines for coronary artery bypass graft surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for Coronary Artery Bypass Graft Surgery). J Am Coll Cardiol 1999;34:1262-347.
- ↑ Goldman S, Copeland J, Moritz T, Henderson W, Zadina K, Ovitt T, Kern KB, Sethi G, Sharma GV, Khuri S (1991). "Starting aspirin therapy after operation. Effects on early graft patency. Department of Veterans Affairs Cooperative Study Group". Circulation. 84 (2): 520–6. PMID 1860197. Retrieved 2010-07-22. Unknown parameter
|month=ignored (help) - ↑ Patel JH, Stoner JA, Owora A, Mathew ST, Thadani U (2009). "Evidence for using clopidogrel alone or in addition to aspirin in post coronary artery bypass surgery patients". Am. J. Cardiol. 103 (12): 1687–93. doi:10.1016/j.amjcard.2009.02.021. PMID 19539077. Retrieved 2010-07-22. Unknown parameter
|month=ignored (help) - ↑ Savage MP, Douglas JS, Fischman DL; et al. (1997). "Stent placement compared with balloon angioplasty for obstructed coronary bypass grafts. Saphenous Vein De Novo Trial Investigators". N. Engl. J. Med. 337 (11): 740–7. PMID 9287229. Unknown parameter
|month=ignored (help) - ↑ Stankovic G, Colombo A, Presbitero P; et al. (2003). "Randomized evaluation of polytetrafluoroethylene-covered stent in saphenous vein grafts: the Randomized Evaluation of polytetrafluoroethylene COVERed stent in Saphenous vein grafts (RECOVERS) Trial". Circulation. 108 (1): 37–42. doi:10.1161/01.CIR.0000079106.71097.1C. PMID 12821546. Unknown parameter
|month=ignored (help) - ↑ Schächinger V, Hamm CW, Münzel T; et al. (2003). "A randomized trial of polytetrafluoroethylene-membrane-covered stents compared with conventional stents in aortocoronary saphenous vein grafts". J. Am. Coll. Cardiol. 42 (8): 1360–9. PMID 14563575. Unknown parameter
|month=ignored (help) - ↑ Salloum J, Tharpe C, Vaughan D, Zhao DX (2005). "Release and elimination of soluble vasoactive factors during percutaneous coronary intervention of saphenous vein grafts: analysis using the PercuSurge GuardWire distal protection device". J Invasive Cardiol. 17 (11): 575–9. PMID 16264199. Unknown parameter
|month=ignored (help) - ↑ Baim DS, Wahr D, George B; et al. (2002). "Randomized trial of a distal embolic protection device during percutaneous intervention of saphenous vein aorto-coronary bypass grafts". Circulation. 105 (11): 1285–90. PMID 11901037. Unknown parameter
|month=ignored (help) - ↑ Stone GW, Rogers C, Hermiller J; et al. (2003). "Randomized comparison of distal protection with a filter-based catheter and a balloon occlusion and aspiration system during percutaneous intervention of diseased saphenous vein aorto-coronary bypass grafts". Circulation. 108 (5): 548–53. doi:10.1161/01.CIR.0000080894.51311.0A. PMID 12874191. Unknown parameter
|month=ignored (help) - ↑ Halkin A, Masud AZ, Rogers C; et al. (2006). "Six-month outcomes after percutaneous intervention for lesions in aortocoronary saphenous vein grafts using distal protection devices: results from the FIRE trial". Am. Heart J. 151 (4): 915.e1–7. doi:10.1016/j.ahj.2005.09.018. PMID 16569562. Unknown parameter
|month=ignored (help) - ↑ Mauri L, Cox D, Hermiller J; et al. (2007). "The PROXIMAL trial: proximal protection during saphenous vein graft intervention using the Proxis Embolic Protection System: a randomized, prospective, multicenter clinical trial". J. Am. Coll. Cardiol. 50 (15): 1442–9. doi:10.1016/j.jacc.2007.06.039. PMID 17919563. Unknown parameter
|month=ignored (help) - ↑ Holmes DR, Topol EJ, Califf RM; et al. (1995). "A multicenter, randomized trial of coronary angioplasty versus directional atherectomy for patients with saphenous vein bypass graft lesions. CAVEAT-II Investigators". Circulation. 91 (7): 1966–74. PMID 7895354. Unknown parameter
|month=ignored (help) - ↑ Zoghbi GJ, Goyal M, Hage F; et al. (2009). "Pretreatment with nitroprusside for microcirculatory protection in saphenous vein graft interventions". J Invasive Cardiol. 21 (2): 34–9. PMID 19182287. Unknown parameter
|month=ignored (help)