Congenital adrenal hyperplasia Overview: Difference between revisions
(Created page with "__NOTOC__ {{Congenital adrenal hyperplasia}} {{CMG}}; {{AE}}{{MJ}} {{SK}} Congenital adrenal hyperplasia, CAH, Adrenal hyperplasia ==Overview== Congenital adrenal hyperplas...") |
|||
| (4 intermediate revisions by the same user not shown) | |||
| Line 10: | Line 10: | ||
Prenatal diagnosis can be made in both of these disorders by chorionic villous sampling, but this can only be done at 8-10 weeks. In order to prevent the deleterious effect of excess androgens on genital (and brain!) development, therapy must be started earlier. This is most often considered if there is an affected sibling. Treatment is dexamethasone, which is not degraded by the placenta, but is associated with significant maternal weight gain, hypertension, and edema. | Prenatal diagnosis can be made in both of these disorders by chorionic villous sampling, but this can only be done at 8-10 weeks. In order to prevent the deleterious effect of excess androgens on genital (and brain!) development, therapy must be started earlier. This is most often considered if there is an affected sibling. Treatment is dexamethasone, which is not degraded by the placenta, but is associated with significant maternal weight gain, hypertension, and edema. | ||
== | ==Classification== | ||
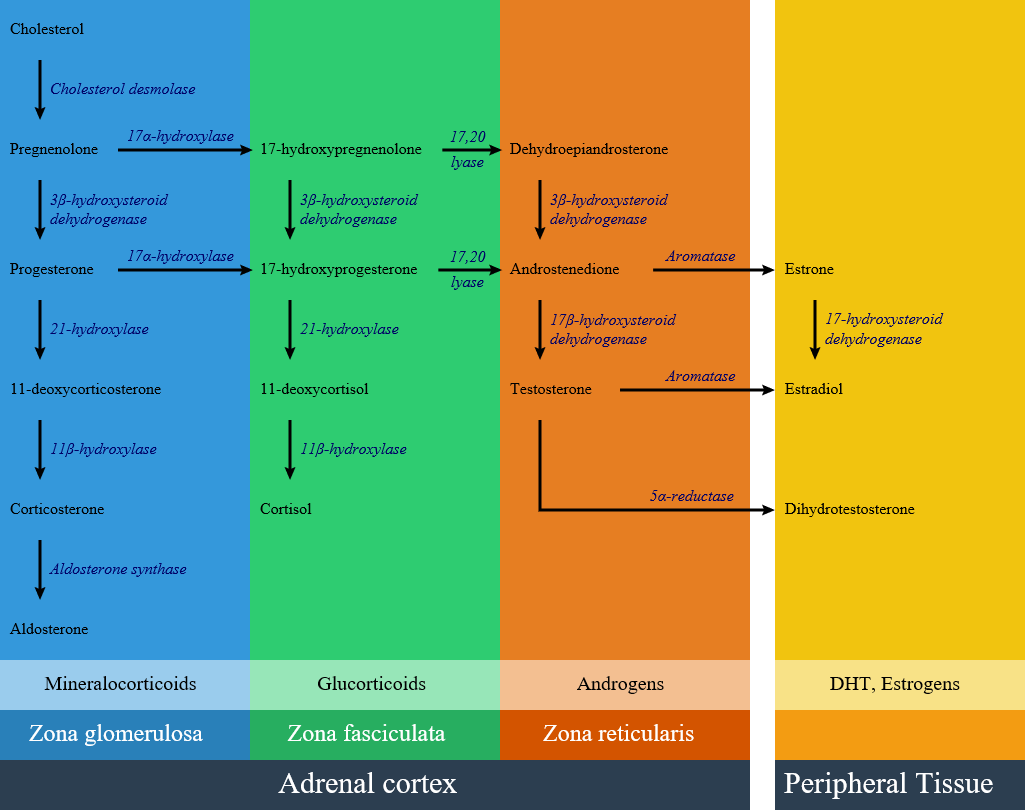
Congenital adrenal hyperplasia is classified into seven types based on the genetic causes that lead to hyperplasia and hormonal imbalance. There are three zones of hormonal synthesis in adrenal cortex that are shown below, and impairment of each pathway may lead to a specific subtype of congenital adrenal hyperplasia. | |||
[[image:Adrenal Steroids.png|600px]] | [[image:Adrenal Steroids.png|600px]] | ||
{| align="center" class="wikitable" style="border: 0px; font-size: 90%; margin: 3px;" | {| align="center" class="wikitable" style="border: 0px; font-size: 90%; margin: 3px;" | ||
! align="center" style="background:#DCDCDC;" rowspan="2" colspan="2" |Disease | ! align="center" style="background:#DCDCDC;" rowspan="2" colspan="2" |Disease | ||
! align="center" style="background:#DCDCDC;" colspan="2" |History and symptoms | ! align="center" style="background:#DCDCDC;" colspan="2" |History and symptoms | ||
! align="center" style="background:#DCDCDC;" colspan=" | ! align="center" style="background:#DCDCDC;" colspan="3" |Laboratory findings | ||
! align="center" style="background:#DCDCDC;" |Defective gene | ! align="center" style="background:#DCDCDC;" |Defective gene | ||
|- | |- | ||
!Blood pressure | !Blood pressure | ||
!Genitalia | !Genitalia | ||
! | !Increased | ||
!Decreased | |||
!K levels | !K levels | ||
! | ! | ||
| Line 40: | Line 39: | ||
* Male: normal or scrotal pigmentation and large phallus | * Male: normal or scrotal pigmentation and large phallus | ||
| | | | ||
* [[Deoxycorticosterone]] | |||
* | * 11-Deoxy-[[cortisol]] | ||
* | * [[17-Hydroxyprogesterone|17-hydroxyprogesterone]], mild elevation | ||
| | |||
* [[Cortisol]] | |||
* [[Corticosterone]] | |||
* | * [[Aldosterone]] | ||
* Corticosterone | |||
* | |||
| | | | ||
* High in salt wasting type | * High in salt wasting type | ||
| Line 61: | Line 58: | ||
* Female: virilization after puberty | * Female: virilization after puberty | ||
* Male: normal appearance | * Male: normal appearance | ||
| | | | ||
* 17- | * [[17-Hydroxyprogesterone|17-hydroxyprogesterone]] | ||
* Exaggerated | * Exaggerated [[Androstenedione]], [[DHEA]], and [[17-Hydroxyprogesterone|17-hydroxyprogesterone]] | ||
response to ACTH | response to [[ACTH]] | ||
| | |||
| | | | ||
* Normal | * Normal | ||
| Line 70: | Line 68: | ||
* CYP21A1 and CYP21A2 gene | * CYP21A1 and CYP21A2 gene | ||
|- | |- | ||
| align="center" style="padding: 5px 5px; background: #DCDCDC;" colspan="2" | | | align="center" style="padding: 5px 5px; background: #DCDCDC;" colspan="2" |[[17 alpha-hydroxylase deficiency|17-α hydroxylase deficiency]] | ||
| align="center" style="padding: 5px 5px; background: #F5F5F5;" | | | align="center" style="padding: 5px 5px; background: #F5F5F5;" | | ||
* Hypertension | * Hypertension | ||
| Line 77: | Line 75: | ||
* Male: ambiguous | * Male: ambiguous | ||
| align="center" style="padding: 5px 5px; background: #F5F5F5;" | | | align="center" style="padding: 5px 5px; background: #F5F5F5;" | | ||
* | * [[Deoxycorticosterone]] | ||
* [[Corticosterone]] | |||
* | * [[Progesterone]] | ||
| | |||
* [[Cortisol]] | |||
* [[Aldosterone]] | |||
| | | | ||
* Low | * Low | ||
| align="center" style="padding: 5px 5px; background: #F5F5F5;" | | | align="center" style="padding: 5px 5px; background: #F5F5F5;" | | ||
* | * CYP17A1 | ||
|- | |- | ||
| align="center" style="padding: 5px 5px; background: #DCDCDC;" colspan="2" |11β-hydroxylase deficiency | | align="center" style="padding: 5px 5px; background: #DCDCDC;" colspan="2" |[[11β-hydroxylase deficiency|11-β hydroxylase deficiency]] | ||
| align="center" style="padding: 5px 5px; background: #F5F5F5;" | | | align="center" style="padding: 5px 5px; background: #F5F5F5;" | | ||
* Hypertension | * Hypertension | ||
| Line 93: | Line 94: | ||
* Male: normal or scrotal pigmentation and large phallus | * Male: normal or scrotal pigmentation and large phallus | ||
| align="center" style="padding: 5px 5px; background: #F5F5F5;" | | | align="center" style="padding: 5px 5px; background: #F5F5F5;" | | ||
* | * [[Deoxycorticosterone]] | ||
* 11-Deoxy-[[cortisol]] | |||
* | * [[17-Hydroxyprogesterone|17-hydroxyprogesterone]], mild elevation | ||
| | |||
* [[Cortisol]] | |||
* [[Corticosterone]] | |||
* [[Aldosterone]] | |||
| | | | ||
* Low | * Low | ||
| align="center" style="padding: 5px 5px; background: #F5F5F5;" | | | align="center" style="padding: 5px 5px; background: #F5F5F5;" | | ||
* | * CYP11B1 | ||
|- | |- | ||
| align="center" style="padding: 5px 5px; background: #DCDCDC;" colspan="2" | | | align="center" style="padding: 5px 5px; background: #DCDCDC;" colspan="2" |3β-Hydroxysteroid Dehydrogenase | ||
| align="center" style="padding: 5px 5px; background: #F5F5F5;" | | | align="center" style="padding: 5px 5px; background: #F5F5F5;" | | ||
| | | | ||
| align="center" style="padding: 5px 5px; background: #F5F5F5;" | | | align="center" style="padding: 5px 5px; background: #F5F5F5;" | | ||
* | * [[Dehydroepiandrosterone]] | ||
* 17- | * [[17-hydroxypregnenolone]] | ||
* Pregnenolone | * [[Pregnenolone]] | ||
| | |||
* Cortisol | * [[Cortisol]] | ||
* Aldosterone | * [[Aldosterone]] | ||
| | | | ||
* High | * High | ||
| Line 119: | Line 124: | ||
| | | | ||
| align="center" style="padding: 5px 5px; background: #F5F5F5;" | | | align="center" style="padding: 5px 5px; background: #F5F5F5;" | | ||
| | |||
| | | | ||
| align="center" style="padding: 5px 5px; background: #F5F5F5;" | | | align="center" style="padding: 5px 5px; background: #F5F5F5;" | | ||
| Line 126: | Line 132: | ||
| | | | ||
| align="center" style="padding: 5px 5px; background: #F5F5F5;" | | | align="center" style="padding: 5px 5px; background: #F5F5F5;" | | ||
| | |||
| | | | ||
| align="center" style="padding: 5px 5px; background: #F5F5F5;" | | | align="center" style="padding: 5px 5px; background: #F5F5F5;" | | ||
| Line 133: | Line 140: | ||
| | | | ||
| align="center" style="padding: 5px 5px; background: #F5F5F5;" | | | align="center" style="padding: 5px 5px; background: #F5F5F5;" | | ||
| | |||
| | | | ||
| align="center" style="padding: 5px 5px; background: #F5F5F5;" | | | align="center" style="padding: 5px 5px; background: #F5F5F5;" | | ||
|} | |} | ||
==Differential Diagnosis== | |||
[[Congenital adrenal hyperplasia]] must be differentiated from diseases that cause [[ambiguous genitalia]]:<ref name="pmid17875484">{{cite journal |vauthors=Hughes IA, Nihoul-Fékété C, Thomas B, Cohen-Kettenis PT |title=Consequences of the ESPE/LWPES guidelines for diagnosis and treatment of disorders of sex development |journal=Best Pract. Res. Clin. Endocrinol. Metab. |volume=21 |issue=3 |pages=351–65 |year=2007 |pmid=17875484 |doi=10.1016/j.beem.2007.06.003 |url=}}</ref><ref name="pmid10857554">{{cite journal |vauthors=White PC, Speiser PW |title=Congenital adrenal hyperplasia due to 21-hydroxylase deficiency |journal=Endocr. Rev. |volume=21 |issue=3 |pages=245–91 |year=2000 |pmid=10857554 |doi=10.1210/edrv.21.3.0398 |url=}}</ref> | |||
{| class="wikitable" | |||
!Disease name | |||
! colspan="2" |Laboratory tests | |||
!Important clinical findings | |||
|- | |||
! | |||
!Increased | |||
!Decreased | |||
! | |||
|- | |||
|[[21-hydroxylase deficiency|Classic type of 21-hydroxylase deficiency]] | |||
| | |||
* [[17-Hydroxyprogesterone|17-hydroxyprogesterone]] | |||
* [[Progesterone]] | |||
* [[Androstenedione]] | |||
* [[DHEA]] | |||
| | |||
* [[Aldosterone]] | |||
* [[Corticosterone]] (salt-wasting) | |||
* [[Cortisol]] (simple [[virilizing]]) | |||
| | |||
* [[Ambiguous genitalia]] in female | |||
* [[Virilization]] in female | |||
* Salt-wasting | |||
* [[Hypotension]] and [[hyperkalemia]] | |||
|- | |||
|[[11β-hydroxylase deficiency|11-β hydroxylase deficiency]] | |||
| | |||
* [[Deoxycorticosterone]] | |||
* 11-Deoxy-[[cortisol]] | |||
* [[17-Hydroxyprogesterone|17-hydroxyprogesterone]], mild elevation | |||
| | |||
* [[Cortisol]] | |||
* [[Corticosterone]] | |||
* [[Aldosterone]] | |||
| | |||
* [[Ambiguous genitalia]] in female | |||
* [[Hypertension]] and [[hypokalemia]] | |||
* [[Virilization]] | |||
|- | |||
|[[17 alpha-hydroxylase deficiency|17-α hydroxylase deficiency]] | |||
| | |||
* [[Deoxycorticosterone]] | |||
* [[Corticosterone]] | |||
* [[Progesterone]] | |||
| | |||
* [[Cortisol]] | |||
* [[Aldosterone]] | |||
| | |||
* [[Ambiguous genitalia]] in male | |||
* [[Hypertension]] | |||
* [[Primary amenorrhea]] | |||
* Absence of [[secondary sexual characteristics]] | |||
* Minimal [[body hair]] | |||
|- | |||
|3β-Hydroxysteroid Dehydrogenase | |||
| | |||
* [[Dehydroepiandrosterone]] | |||
* [[17-hydroxypregnenolone]] | |||
* [[Pregnenolone]] | |||
| | |||
* [[Cortisol]] | |||
* [[Aldosterone]] | |||
| | |||
* [[Vomiting]], [[volume depletion]], [[hyponatremia]], and [[hyperkalemia]] | |||
* 46-XY infants often show [[undervirilization]], due to a block in [[testosterone]] synthesis | |||
|- | |||
|Gestational [[hyperandrogenism]] | |||
| colspan="2" | | |||
* Maternal serum [[androgen]] concentrations (usually [[testosterone]] and [[androstenedione]]) are high | |||
* If [[virilization]] is caused by exogenous hormone administration, the values may be low because the offending hormone is usually a synthetic [[steroid]] not measured in assays for [[testosterone]] or other [[androgens]] | |||
| | |||
* [[Androgen]] excess sign and symptoms in mother | |||
* History of [[androgen]] containing [[medication]] consumption during [[pregnancy]] in mother | |||
* [[Virilization]] in a 46,XX individual with normal female internal anatomy | |||
* Causes include maternal [[luteoma]] or theca-[[lutein]] [[cysts]], and [[placental]] [[aromatase]] enzyme deficiency | |||
|} | |||
congenital adrenal hyperplasia must be differentiated from diseases that cause [[virilization]] and [[hirsutism]] in female:<ref name="pmid24830586">{{cite journal |vauthors=Hohl A, Ronsoni MF, Oliveira Md |title=Hirsutism: diagnosis and treatment |journal=Arq Bras Endocrinol Metabol |volume=58 |issue=2 |pages=97–107 |year=2014 |pmid=24830586 |doi= |url=}}</ref><ref name="pmid10857554">{{cite journal |vauthors=White PC, Speiser PW |title=Congenital adrenal hyperplasia due to 21-hydroxylase deficiency |journal=Endocr. Rev. |volume=21 |issue=3 |pages=245–91 |year=2000 |pmid=10857554 |doi=10.1210/edrv.21.3.0398 |url=}}</ref><ref name="ISBN:978-0323297387">{{cite book | last = Melmed | first = Shlomo | title = Williams textbook of endocrinology | publisher = Elsevier | location = Philadelphia, PA | year = 2016 | isbn = 978-0323297387 }}=</ref> | |||
{| class="wikitable" | |||
!Disease name | |||
!Steroid status | |||
!Other laboratory | |||
!Important clinical findings | |||
|- | |||
|Non-classic type of 21-hydroxylase deficiency | |||
|Increased: | |||
* [[17-Hydroxyprogesterone|17-hydroxyprogesterone]] | |||
* Exaggerated [[Androstenedione]], [[DHEA]], and [[17-Hydroxyprogesterone|17-hydroxyprogesterone]] | |||
response to [[ACTH]] | |||
| | |||
* Low [[testosterone]] levels | |||
| | |||
* No symptoms in infancy and male | |||
* [[Virilization]] in females | |||
|- | |||
|[[11β-hydroxylase deficiency|11-β hydroxylase deficiency]] | |||
|Increased: | |||
* DOC | |||
* 11-Deoxy-[[Cortisol]] | |||
Decreased: | |||
* [[Cortisol]] | |||
* [[Corticosterone]] | |||
* [[Aldosterone]] | |||
| | |||
* Low [[testosterone]] levels | |||
| | |||
* [[Hypertension]] and [[hypokalemia]] | |||
* [[Virilization]] | |||
|- | |||
|3β-Hydroxysteroid Dehydrogenase | |||
|Increased: | |||
* [[DHEA]] | |||
* [[17-hydroxypregnenolone]] | |||
* [[Pregnenolone]] | |||
Decreased: | |||
* [[Cortisol]] | |||
* [[Aldosterone]] | |||
| | |||
* Low [[testosterone]] levels | |||
| | |||
* Salt-wasting [[adrenal crisis]] in infancy | |||
* Mild [[virilization]] of genetically female infants | |||
* [[Undervirilization]] of genetically male infants, making it the only form of [[CAH]] which can cause [[ambiguous genitalia]] in both genetic sexes. | |||
|- | |||
|[[Polycystic ovary syndrome ]] | |||
| | |||
* High [[DHEAS]] and [[androstenedione]] levels | |||
| | |||
* Low [[testosterone]] levels | |||
| | |||
* [[Polycystic ovaries]] in sonography | |||
* [[Obesity]] | |||
* [[PCOS]] is the most common cause of [[hirsutism]] in women | |||
* No evidence another diagnosis | |||
|- | |||
|[[Adrenal tumors]] | |||
| | |||
* Variable levels depends on [[tumor]] type | |||
| | |||
* Low [[testosterone]] level | |||
| | |||
* Older age | |||
* Rapidly progressive symptoms | |||
|- | |||
|Ovarian [[virilizing]] tumor | |||
| | |||
* Variable levels depends on [[tumor]] type | |||
| | |||
* [[Testosterone]] is high | |||
| | |||
* Older age | |||
* Rapidly progressive symptoms | |||
|- | |||
|[[Cushing's syndrome]] | |||
| | |||
* Increase [[cortisol]] & metabolites | |||
* Variable other [[steroids]] | |||
| | |||
* Variable [[mineralocorticoid]] excess | |||
| | |||
* [[Cushingoid appearance]] | |||
|- | |||
|[[Hyperprolactinemia]] | |||
| | |||
* Normal levels of most of [[steroids]] | |||
| | |||
* Increased [[prolactin]] | |||
| | |||
* [[Infertility]], [[galactorrhea]] | |||
|} | |||
== | ==Screening== | ||
According to Endocrine Society Clinical Practice Guideline, screening for 21-hydroxylase deficiency by measuring 17a-hydroxyprogesterone is recommended for all newborns. | |||
*Blood sample on filter paper should be obtained from heel puncture preferably between two and four days after birth. | |||
*Screening programs should be done using a two-tier protocol (initial immunoassay with further evaluation of positive tests by liquid chromatography/tandem mass spectrometry. | |||
*Most affected neonates have concentrations greater than 3500 ng/dL (105 nmol/L).<ref name="pmid2208708">{{cite journal |vauthors=Gonzalez RR, Mäentausta O, Solyom J, Vihko R |title=Direct solid-phase time-resolved fluoroimmunoassay of 17 alpha-hydroxyprogesterone in serum and dried blood spots on filter paper |journal=Clin. Chem. |volume=36 |issue=9 |pages=1667–72 |year=1990 |pmid=2208708 |doi= |url=}}</ref><ref name="pmid20823466">{{cite journal |vauthors=Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, Meyer-Bahlburg HF, Miller WL, Montori VM, Oberfield SE, Ritzen M, White PC |title=Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline |journal=J. Clin. Endocrinol. Metab. |volume=95 |issue=9 |pages=4133–60 |year=2010 |pmid=20823466 |pmc=2936060 |doi=10.1210/jc.2009-2631 |url=}}</ref> | |||
===Genetic counseling=== | |||
The Endocrine Society's Clinical Practice Guideline recommends that genetic counseling be provided for individuals who are planning to conceive, and there is a family history of 21-hydroxylase deficiency.<ref name="pmid20823466">{{cite journal |vauthors=Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, Meyer-Bahlburg HF, Miller WL, Montori VM, Oberfield SE, Ritzen M, White PC |title=Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline |journal=J. Clin. Endocrinol. Metab. |volume=95 |issue=9 |pages=4133–60 |year=2010 |pmid=20823466 |pmc=2936060 |doi=10.1210/jc.2009-2631 |url=}}</ref> | |||
==References== | ==References== | ||
{{reflist|2}} | {{reflist|2}} | ||
Latest revision as of 19:26, 1 August 2017
|
Congenital adrenal hyperplasia main page |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mehrian Jafarizade, M.D [2]
Synonyms and keywords: Congenital adrenal hyperplasia, CAH, Adrenal hyperplasia
Overview
Congenital adrenal hyperplasia (CAH) refers to any of several autosomal recessive conditions resulting from biochemical paths of the steroidogenesis of cortisol from cholesterol by the adrenal glands. Most of these conditions involve greater or lesser production of sex steroids and can alter development of primary or secondary sex characteristics in affected infants, children, and adults. Only a small minority of people with CAH can be said to have an intersex condition, but this attracted American public attention in the late 1990s and many accounts of varying accuracy have appeared in the popular media. Approximately 95% of cases of CAH are due to 21-hydroxylase deficiency. Prenatal diagnosis can be made in both of these disorders by chorionic villous sampling, but this can only be done at 8-10 weeks. In order to prevent the deleterious effect of excess androgens on genital (and brain!) development, therapy must be started earlier. This is most often considered if there is an affected sibling. Treatment is dexamethasone, which is not degraded by the placenta, but is associated with significant maternal weight gain, hypertension, and edema.
Classification
Congenital adrenal hyperplasia is classified into seven types based on the genetic causes that lead to hyperplasia and hormonal imbalance. There are three zones of hormonal synthesis in adrenal cortex that are shown below, and impairment of each pathway may lead to a specific subtype of congenital adrenal hyperplasia.
| Disease | History and symptoms | Laboratory findings | Defective gene | ||||
|---|---|---|---|---|---|---|---|
| Blood pressure | Genitalia | Increased | Decreased | K levels | |||
| 21-hydroxylase deficiency | Classic type |
|
|
|
|
| |
| Non-classic type |
|
|
response to ACTH |
|
| ||
| 17-α hydroxylase deficiency |
|
|
|
| |||
| 11-β hydroxylase deficiency |
|
|
|
|
| ||
| 3β-Hydroxysteroid Dehydrogenase |
|
||||||
| Cytochrome P450-oxidoreductase (POR) deficiency (ORD) | |||||||
| Congenital lipoid adrenal hyperplasia | |||||||
| Cholesterol side-chain cleavage enzyme deficiency | |||||||
Differential Diagnosis
Congenital adrenal hyperplasia must be differentiated from diseases that cause ambiguous genitalia:[1][2]
| Disease name | Laboratory tests | Important clinical findings | |
|---|---|---|---|
| Increased | Decreased | ||
| Classic type of 21-hydroxylase deficiency |
|
| |
| 11-β hydroxylase deficiency |
|
| |
| 17-α hydroxylase deficiency |
| ||
| 3β-Hydroxysteroid Dehydrogenase |
| ||
| Gestational hyperandrogenism |
|
| |
congenital adrenal hyperplasia must be differentiated from diseases that cause virilization and hirsutism in female:[3][2][4]
| Disease name | Steroid status | Other laboratory | Important clinical findings |
|---|---|---|---|
| Non-classic type of 21-hydroxylase deficiency | Increased:
response to ACTH |
|
|
| 11-β hydroxylase deficiency | Increased:
Decreased: |
|
|
| 3β-Hydroxysteroid Dehydrogenase | Increased:
Decreased: |
|
|
| Polycystic ovary syndrome |
|
|
|
| Adrenal tumors |
|
|
|
| Ovarian virilizing tumor |
|
|
|
| Cushing's syndrome |
|
||
| Hyperprolactinemia |
|
|
Screening
According to Endocrine Society Clinical Practice Guideline, screening for 21-hydroxylase deficiency by measuring 17a-hydroxyprogesterone is recommended for all newborns.
- Blood sample on filter paper should be obtained from heel puncture preferably between two and four days after birth.
- Screening programs should be done using a two-tier protocol (initial immunoassay with further evaluation of positive tests by liquid chromatography/tandem mass spectrometry.
- Most affected neonates have concentrations greater than 3500 ng/dL (105 nmol/L).[5][6]
Genetic counseling
The Endocrine Society's Clinical Practice Guideline recommends that genetic counseling be provided for individuals who are planning to conceive, and there is a family history of 21-hydroxylase deficiency.[6]
References
- ↑ Hughes IA, Nihoul-Fékété C, Thomas B, Cohen-Kettenis PT (2007). "Consequences of the ESPE/LWPES guidelines for diagnosis and treatment of disorders of sex development". Best Pract. Res. Clin. Endocrinol. Metab. 21 (3): 351–65. doi:10.1016/j.beem.2007.06.003. PMID 17875484.
- ↑ 2.0 2.1 White PC, Speiser PW (2000). "Congenital adrenal hyperplasia due to 21-hydroxylase deficiency". Endocr. Rev. 21 (3): 245–91. doi:10.1210/edrv.21.3.0398. PMID 10857554.
- ↑ Hohl A, Ronsoni MF, Oliveira M (2014). "Hirsutism: diagnosis and treatment". Arq Bras Endocrinol Metabol. 58 (2): 97–107. PMID 24830586. Vancouver style error: initials (help)
- ↑ Melmed, Shlomo (2016). Williams textbook of endocrinology. Philadelphia, PA: Elsevier. ISBN 978-0323297387.=
- ↑ Gonzalez RR, Mäentausta O, Solyom J, Vihko R (1990). "Direct solid-phase time-resolved fluoroimmunoassay of 17 alpha-hydroxyprogesterone in serum and dried blood spots on filter paper". Clin. Chem. 36 (9): 1667–72. PMID 2208708.
- ↑ 6.0 6.1 Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, Meyer-Bahlburg HF, Miller WL, Montori VM, Oberfield SE, Ritzen M, White PC (2010). "Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline". J. Clin. Endocrinol. Metab. 95 (9): 4133–60. doi:10.1210/jc.2009-2631. PMC 2936060. PMID 20823466.