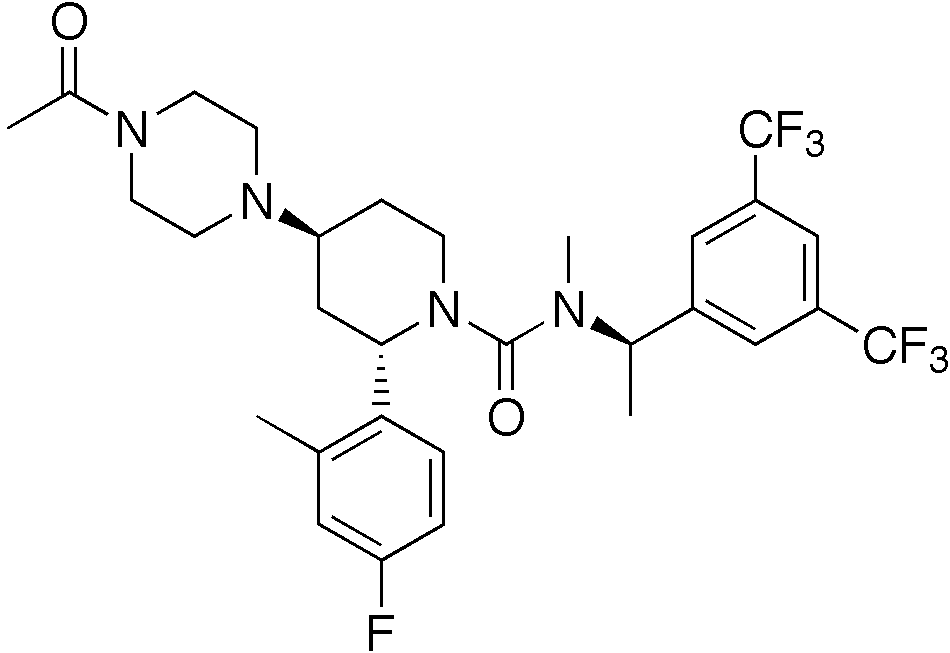
Casopitant
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C30H35F7N4O2 |
| Molar mass | 616.26 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Casopitant (trade names Rezonic (US), Zunrisa (EU)) is an neurokinin 1 (NK1) receptor antagonist undergoing research for the treatment of chemotherapy-induced nausea and vomiting (CINV).[1] It is currently under development by GlaxoSmithKline (GSK).
In July 2008, the company filed a marketing authorisation application with the European Medicines Agency. The application was withdrawn in September 2009 because GSK decided that further safety assessment was necessary.[2]
See also
References
- ↑ Lohr L (2008). "Chemotherapy-induced nausea and vomiting". Cancer J. 14 (2): 85–93. doi:10.1097/PPO.0b013e31816a0f07. PMID 18391612.
- ↑ "GlaxoSmithKline withdraws its marketing authorisation application for Zunrisa" (PDF). London: EMEA. 13 October 2009. Retrieved 21 December 2009.
Template:Antiemetics and antinauseants Template:Antidepressants Template:Anxiolytics Template:Neuropeptide agonists and antagonists
Template:Gastrointestinal-drug-stub
Template:Nervous-system-drug-stub
Categories:
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugs with no legal status
- Drugboxes which contain changes to watched fields
- Pages using div col with unknown parameters
- Antiemetics
- NK1 receptor antagonists
- Amides
- Piperazines
- Piperidines
- Organofluorides