Calcitonin (nasal)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Calcitonin (nasal) is an endocrine metabolic agent that is FDA approved for the treatment of postmenopausal osteoporosis in women greater than 5 years postmenopause when alternative treatments are not suitable. Common adverse reactions include rhinitis, epistaxis, back pain, arthralgia, and headache..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Treatment of Postmenopausal Osteoporosis
- Fortical nasal spray is indicated for the treatment of postmenopausal osteoporosis in women greater than 5 years postmenopause. Fracture reduction efficacy has not been demonstrated. Fortical nasal spray should be reserved for patients for whom alternative treatments are not suitable (e.g., patients for whom other therapies are contraindicated or for patients who are intolerant or unwilling to use other therapies).
Important Limitations of Use
- Due to the possible association between malignancy and calcitonin-salmon use, the need for continued therapy should be re-evaluated on a periodic basis.
Calcitonin-salmon nasal spray has not been shown to increase spinal bone mineral density in early postmenopausal women.
Dosing
Basic Dosing Information
- The recommended dose of Fortical nasal spray is 1 spray (200 International Units) per day intranasally, alternating nostrils daily.
Priming (Activation) of Pump
- Unopened Fortical nasal spray should be stored in the refrigerator. Before using the first dose of Fortical nasal spray, the patient should wait until the bottle has reached room temperature. Remove the protective cap and clip from the bottle of Fortical nasal spray. To prime the pump before it is used for the first time, the bottle should be held upright and the two white side arms of the pump depressed toward the bottle at least 5 times until a full spray is produced. The pump is primed once the first full spray is emitted. To administer, the nozzle should be carefully placed into the nostril with the patient's head in the upright position, then the pump should be firmly depressed toward the bottle. The pump should not be primed before each daily use.
Recommendations for Calcium and Vitamin D Supplementation
- Patients who use Fortical nasal spray should receive adequate calcium (at least 1000 mg elemental calcium per day) and Vitamin D (at least 400 International Units per day).
DOSAGE FORMS AND STRENGTHS
- Fortical nasal spray consists of one glass bottle and one screw-on pump. The bottle contains 3.7 mL of calcitonin-salmon clear solution at a concentration of 2200 International Units per mL. A primed pump delivers 0.09 mL (200 International Units) calcitonin-salmon per actuation.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Calcitonin (nasal) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Calcitonin (nasal) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Calcitonin (nasal) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Calcitonin (nasal) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Calcitonin (nasal) in pediatric patients.
Contraindications
- Hypersensitivity to calcitonin-salmon or any of the excipients. Reactions have included anaphylactic shock, anaphylaxis, bronchospasm, and swelling of the tongue or throat.
Warnings
Hypersensitivity Reactions
- Serious hypersensitivity reactions have been reported in patients receiving calcitonin-salmon nasal spray, e.g., bronchospasm, swelling of the tongue or throat, anaphylaxis and anaphylactic shock. Reports of serious hypersensitivity reactions with injectable calcitonin-salmon have also been reported, including reports of death attributed to anaphylaxis. The usual provisions should be made for emergency treatment if such a reaction occurs. Hypersensitivity reactions should be differentiated from generalized flushing and hypotension.
- For patients with suspected hypersensitivity to calcitonin-salmon, skin testing should be considered prior to treatment utilizing a dilute, sterile solution of a calcitonin-salmon injectable product. Healthcare providers may wish to refer patients who require skin testing to an allergist. A detailed skin testing protocol is available from Upsher-Smith Laboratories, Inc. by calling toll-free at 1-888-650-3789.
Hypocalcemia
- Hypocalcemia associated with tetany (i.e. muscle cramps, twitching) and seizure activity has been reported with calcitonin therapy. Hypocalcemia must be corrected before initiating therapy with Fortical nasal spray. Other disorders affecting mineral metabolism (such as vitamin D deficiency) should also be effectively treated. In patients with these conditions, serum calcium and symptoms of hypocalcemia should be monitored during therapy with Fortical nasal spray. Use of Fortical nasal spray is recommended in conjunction with an adequate intake of calcium and vitamin D.
Nasal Adverse Reactions
- Adverse reactions related to the nose including rhinitis and epistaxis have been reported. Development of mucosal alterations may occur. Therefore, periodic nasal examinations with visualization of the nasal mucosa, turbinates, septum and mucosal blood vessels are recommended prior to start of treatment with Fortical nasal spray, periodically during the course of therapy, and at any time nasal symptoms occur.
- Fortical nasal spray should be discontinued if severe ulceration of the nasal mucosa occurs, as indicated by ulcers greater than 1.5 mm in diameter or penetrating below the mucosa, or those associated with heavy bleeding. Although smaller ulcers often heal without withdrawal of Fortical nasal spray, medication should be discontinued temporarily until healing occurs.
Malignancy
- In a meta-analysis of 21 randomized, controlled clinical trials with calcitonin-salmon (nasal spray or investigational oral formulations), the overall incidence of malignancies reported was higher among calcitonin-salmon-treated patients (4.1%) compared with placebo-treated patients (2.9%). This suggests an increased risk of malignancies in calcitonin-salmon-treated patients compared to placebo-treated patients. The benefits for the individual patient should be carefully considered against possible risks.
Antibody Formation
- Circulating antibodies to calcitonin-salmon have been reported with calcitonin-salmon nasal spray. The possibility of antibody formation should be considered in any patient with an initial response to Fortical nasal spray who later stops responding to treatment.
Urine Sediment Abnormalities
- Coarse granular casts and casts containing renal tubular epithelial cells were reported in young adult volunteers at bed rest who were given injectable calcitonin-salmon to study the effect of immobilization on osteoporosis. There was no other evidence of renal abnormality and the urine sediment normalized after calcitonin-salmon was stopped. Periodic examinations of urine sediment should be considered. Urine sediment abnormalities have not been reported in ambulatory volunteers treated with calcitonin-salmon nasal spray.
Adverse Reactions
Clinical Trials Experience
- The following serious adverse reactions are discussed in greater detail in other sections of the label:
- Hypersensitivity Reactions, including anaphylaxis
- Hypocalcemia
- Nasal Adverse Reactions
- Malignancy
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety of calcitonin-salmon nasal spray in the treatment of postmenopausal osteoporosis was assessed in 5 randomized, double-blind, placebo controlled trials that enrolled postmenopausal women, aged 45-75 years. The duration of the trials ranged from 1 to 2 years. The incidence of adverse reactions reported in studies involving postmenopausal osteoporotic patients chronically exposed to calcitonin-salmon nasal spray (N=341) and to placebo nasal spray (N=131), and reported in greater than 3% of calcitonin-salmon treated patients are presented in the following table. Other than flushing, nausea, possible allergic reactions, and possible local irritative effects in the respiratory tract, a relationship to calcitonin-salmon nasal spray has not been established.
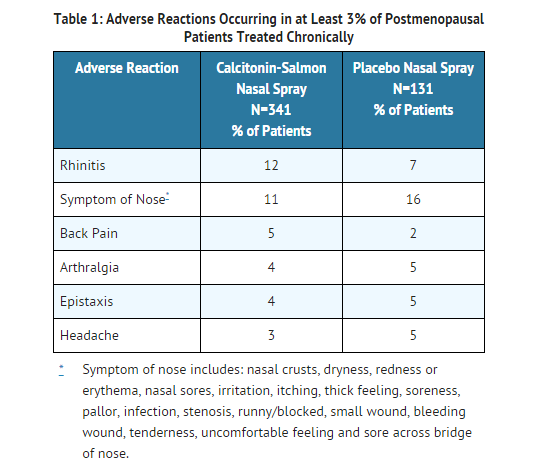
Nasal Adverse Reactions: In all postmenopausal patients treated with calcitonin-salmon nasal spray, the most commonly reported nasal adverse reactions included rhinitis (12%), epistaxis (4%), and sinusitis (2%). Smoking did not have a contributory effect on the occurrence of nasal adverse reactions.
- Adverse reactions reported in 1-3% of patients treated with calcitonin-salmon nasal spray include: influenza-like symptoms, erythematous rash, arthrosis, myalgia, sinusitis, upper respiratory tract infection, bronchospasm, abdominal pain, nausea, dizziness, paresthesia, abnormal lacrimation, conjunctivitis, lymphadenopathy, infection, and depression
Malignancy
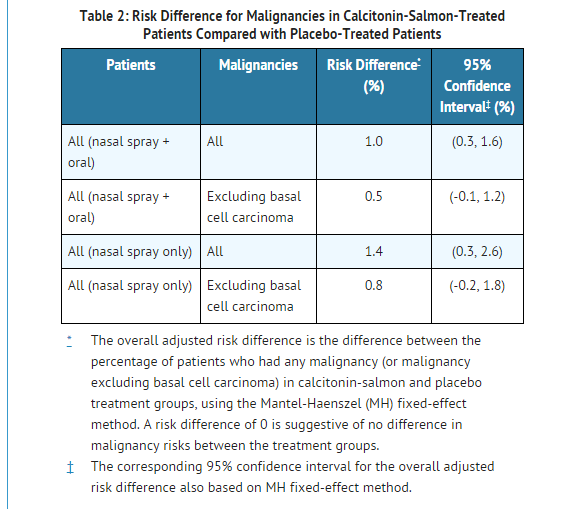
- A meta-analysis of 21 randomized, controlled clinical trials with calcitonin-salmon (nasal spray or investigational oral formulations) was conducted to assess the risk of malignancies in calcitonin-salmon-treated patients compared to placebo-treated patients. The trials in the meta-analysis ranged in duration from 6 months to 5 years and included a total of 10883 patients (6151 treated with calcitonin-salmon and 4732 treated with placebo). The overall incidence of malignancies reported in these 21 trials was higher among calcitonin-salmon-treated patients (254/6151 or 4.1%) compared with placebo-treated patients (137/4732 or 2.9%). Findings were similar when analyses were restricted to the 18 nasal spray only trials [calcitonin-salmon 122/2712 (4.5%); placebo 30/1309 (2.3%)].
- The meta-analysis results suggest an increased risk of overall malignancies in calcitonin-salmon-treated patients compared to placebo-treated patients when all 21 trials are included and when the analysis is restricted to the 18 nasal spray only trials (see TABLE 2). It is not possible to exclude an increased risk when calcitonin-salmon is administered by the subcutaneous, intramuscular, or intravenous route because these routes of administration were not investigated in the meta-analysis. The increased malignancy risk seen with the meta-analysis was heavily influenced by a single large 5-year trial, which had an observed risk difference of 3.4% [95% CI (0.4%, 6.5%)]. Imbalances in risks were still observed when analyses excluded basal cell carcinoma (see TABLE 2); the data were not sufficient for further analyses by type of malignancy. A mechanism for these observations has not been identified. Although a definitive causal relationship between calcitonin-salmon use and malignancies cannot be established from this meta-analysis, the benefits for the individual patient should be carefully evaluated against all possible risks.
Postmarketing Experience
- Because postmarketing adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- The following adverse reactions have been reported during post-approval use of calcitonin-salmon nasal spray.
- Allergic / Hypersensitivity Reactions: Serious allergic reactions have been reported in patients receiving calcitonin-salmon nasal spray, including anaphylaxis and anaphylactic shock.
- Hypocalcemia: Hypocalcemia with paresthesia has been reported.
- Body as a whole: facial or peripheral edema
- Cardiovascular: hypertension, vasodilatation, syncope, chest pain
- Respiratory/ Special Senses: cough, bronchospasm, dyspnea, loss of taste/smell
- Skin: rash/dermatitis, pruritus, alopecia, increased sweating
- Gastrointestinal: diarrhea
- Nervous system disorders: tremor
Drug Interactions
- No formal drug interaction studies have been performed with calcitonin-salmon nasal spray.
- Concomitant use of calcitonin-salmon and lithium may lead to a reduction in plasma lithium concentrations due to increased urinary clearance of lithium. The dose of lithium may require adjustment.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Pregnancy Category C:
Risk Summary
- There are no adequate and well-controlled studies in pregnant women. Fortical nasal spray should be used during pregnancy only if the potential benefit justifies the use as compared with potential risks to the patient and fetus. Based on animal data, Fortical is predicted to have low probability of increasing the risk of adverse developmental outcomes above background risk.
Animal Data
- Synthetic calcitonin-salmon has been shown to cause a decrease in fetal birth weights in rabbits when given by subcutaneous injection at doses 70-278 times the intranasal dose recommended for human use based on body surface area.
- No embryo/fetal toxicities related to synthetic calcitonin-salmon were reported from maternal subcutaneous daily doses in rats up to 80 International Units/kg/day from gestation day 6 to 15.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Calcitonin (nasal) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Calcitonin (nasal) during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. No studies have been conducted to assess the impact of Fortical on milk production in humans, its presence in human breast milk, or its effects on the breast-fed child. Because many drugs are excreted in human milk, caution should be exercised when Fortical is administered to a nursing woman. Synthetic calcitonin-salmon has been shown to inhibit lactation in rats.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- In a multi-centered, double-blind, randomized clinical study of calcitonin-salmon nasal spray, 279 patients were less than 65 years old, while 467 patients were 65 to 74 years old and 196 patients were 75 and over. Compared to subjects less than 65 years old, the incidence of nasal adverse reactions (rhinitis, irritation, erythema, and excoriation) was higher in patients over the age of 65, particularly those over the age of 75. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Gender
There is no FDA guidance on the use of Calcitonin (nasal) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Calcitonin (nasal) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Calcitonin (nasal) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Calcitonin (nasal) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Calcitonin (nasal) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Calcitonin (nasal) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Nasal
Monitoring
There is limited information regarding Calcitonin (nasal) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Calcitonin (nasal) and IV administrations.
Overdosage
- The pharmacologic actions of Fortical nasal spray suggest that hypocalcemic tetany could occur in overdose. Therefore, provisions for parenteral administration of calcium should be available for the treatment of overdose.
- Single doses of calcitonin-salmon nasal spray up to 1600 International Units, doses up to 800 International Units per day for 3 days and chronic administration of doses up to 600 International Units per day have been studied without serious adverse effects.
Pharmacology
There is limited information regarding Calcitonin (nasal) Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Calcitonin (nasal) Mechanism of Action in the drug label.
Structure
There is limited information regarding Calcitonin (nasal) Structure in the drug label.
Pharmacodynamics
There is limited information regarding Calcitonin (nasal) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Calcitonin (nasal) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Calcitonin (nasal) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Calcitonin (nasal) Clinical Studies in the drug label.
How Supplied
There is limited information regarding Calcitonin (nasal) How Supplied in the drug label.
Storage
There is limited information regarding Calcitonin (nasal) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Calcitonin (nasal) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Calcitonin (nasal) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Calcitonin (nasal) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Calcitonin (nasal) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Calcitonin (nasal) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Calcitonin (nasal) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.